The Radical Below Can Be Stabilized By Resonance.
Holbox
Mar 30, 2025 · 5 min read
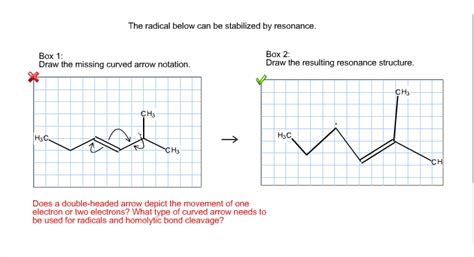
Table of Contents
- The Radical Below Can Be Stabilized By Resonance.
- Table of Contents
- The Radical Below Can Be Stabilized by Resonance: A Deep Dive into Resonance Stabilization
- Understanding Resonance and its Impact on Radical Stability
- Key Factors Influencing Resonance Stabilization in Radicals:
- Examples of Resonance-Stabilized Radicals
- 1. Allyl Radical: A Classic Example
- 2. Benzyl Radical: Aromatic Resonance
- 3. Phenoxyl Radical: Oxygen's Role
- 4. Allylic and Benzylic Radicals: Common Themes
- Consequences of Resonance Stabilization: Reactivity and Stability
- Resonance Stabilization in Different Chemical Contexts
- Advanced Concepts and Further Exploration
- Conclusion: The Importance of Understanding Resonance Stabilization
- Latest Posts
- Latest Posts
- Related Post
The Radical Below Can Be Stabilized by Resonance: A Deep Dive into Resonance Stabilization
Resonance stabilization is a crucial concept in organic chemistry, explaining the unusual stability of certain molecules and radicals. This article will delve deep into the phenomenon, focusing specifically on how resonance contributes to the stability of radicals. We'll explore the underlying principles, provide numerous examples, and discuss the implications of resonance stabilization in various chemical reactions and contexts.
Understanding Resonance and its Impact on Radical Stability
Before we dive into specific examples, let's establish a firm understanding of resonance. Resonance isn't about a molecule rapidly switching between different structures; instead, it represents a single molecule whose properties are a blend of several contributing structures (resonance contributors or canonical forms). These contributing structures are drawn with differing locations of electrons, particularly pi electrons and lone pairs, but the actual molecule exists as a hybrid – a weighted average of these contributing structures.
What makes a radical stable? Radicals, possessing an unpaired electron, are inherently reactive. However, certain factors can significantly enhance their stability. Resonance is one of the most powerful of these factors. The unpaired electron in a resonance-stabilized radical is delocalized across multiple atoms, effectively lowering its energy and making it less reactive compared to a non-resonance-stabilized radical. This delocalization spreads out the electron density, reducing the concentration of negative charge in any one location and thus increasing overall stability.
Key Factors Influencing Resonance Stabilization in Radicals:
-
The number of contributing resonance structures: More contributing structures generally indicate greater stability. A larger number implies a more significant delocalization of the unpaired electron.
-
The stability of individual resonance structures: Structures with filled octets and minimal charge separation are more stable and contribute more significantly to the resonance hybrid.
-
The extent of electron delocalization: The more extensive the delocalization, the greater the stabilization. This is directly tied to the number and nature of conjugated pi systems.
Examples of Resonance-Stabilized Radicals
Let's examine several examples illustrating how resonance stabilizes radicals. We will focus on the impact of different functional groups and molecular frameworks on the extent of stabilization.
1. Allyl Radical: A Classic Example
The allyl radical (CH₂=CH-CH₂) is a classic example of a resonance-stabilized radical. The unpaired electron can be delocalized across the three carbon atoms. This leads to two major resonance contributors:
[ CH₂-CH=CH₂ • ] <--> [ CH₂=CH-CH₂ • ]
The actual structure is a hybrid, with the unpaired electron spread across both terminal carbons. This delocalization significantly increases the stability of the allyl radical compared to a simple alkyl radical like the methyl radical (•CH₃).
2. Benzyl Radical: Aromatic Resonance
The benzyl radical (C₆H₅CH₂) is even more stable due to the involvement of the aromatic ring. The unpaired electron can delocalize into the benzene ring, leading to multiple resonance structures. The resonance stabilization in the benzyl radical is significantly greater than in the allyl radical because of the increased number of contributing structures and the aromatic nature of the ring.
(Several resonance structures with the unpaired electron located at different carbon atoms of the benzene ring would be shown here graphically. This is difficult to represent in markdown, but the reader should envision the electron moving around the ring.)
3. Phenoxyl Radical: Oxygen's Role
The phenoxyl radical, derived from phenol (C₆H₅OH), showcases the influence of a heteroatom (oxygen) on resonance stabilization. The unpaired electron is delocalized across the benzene ring and the oxygen atom, leading to enhanced stability. The oxygen atom's lone pairs participate in the resonance, further extending the delocalization.
4. Allylic and Benzylic Radicals: Common Themes
Allylic radicals (radicals adjacent to a carbon-carbon double bond) and benzylic radicals (radicals adjacent to a benzene ring) are consistently more stable than their non-resonance-stabilized counterparts. This increased stability is directly attributed to the resonance effect. They participate in numerous reactions and are critical intermediates in many organic transformations.
Consequences of Resonance Stabilization: Reactivity and Stability
The enhanced stability resulting from resonance stabilization has significant consequences for the reactivity of these radicals.
-
Reduced Reactivity: Resonance-stabilized radicals are significantly less reactive than their non-stabilized counterparts. They are less likely to undergo rapid reactions like hydrogen abstraction or dimerization.
-
Selectivity in Reactions: The delocalization of the unpaired electron can influence the regioselectivity and stereoselectivity of reactions involving these radicals.
Resonance Stabilization in Different Chemical Contexts
Resonance stabilization plays a crucial role in numerous chemical contexts:
-
Free Radical Polymerization: Resonance-stabilized radicals are often used in free radical polymerization because of their stability and ability to propagate the chain reaction.
-
Organic Synthesis: Understanding resonance stabilization is crucial for designing and predicting the outcome of many organic reactions. The stability of intermediate radicals often dictates the reaction pathway.
-
Biochemistry: Resonance stabilization plays a role in the stability and reactivity of various biological molecules, including radicals involved in enzymatic reactions and metabolic processes.
Advanced Concepts and Further Exploration
The concept of resonance stabilization can be further explored with more advanced concepts:
-
Quantitative assessment of resonance stabilization: Various computational methods can provide quantitative measures of resonance energy and stability.
-
Resonance energy: This term quantifies the additional stability gained due to resonance. It represents the difference in energy between the actual molecule and the most stable contributing structure.
-
Hyperconjugation: This phenomenon, related but distinct from resonance, involves the interaction of sigma bonds with an adjacent empty or partially filled orbital, and can contribute to radical stability.
Conclusion: The Importance of Understanding Resonance Stabilization
Resonance stabilization is a powerful effect that dramatically influences the stability and reactivity of radicals. Understanding this phenomenon is essential for comprehending various aspects of organic chemistry, including reaction mechanisms, synthetic strategies, and the properties of organic molecules. The examples provided here only scratch the surface; the diversity of molecules exhibiting resonance stabilization is vast. Further exploration into this topic reveals its profound impact on the broader field of chemistry. By grasping the fundamental principles of resonance and its impact on radical stability, a deeper understanding of organic reactions and molecular behavior can be achieved. This knowledge is invaluable for students and professionals alike in fields ranging from pharmaceutical chemistry to materials science.
Latest Posts
Latest Posts
-
Problems In Balance May Follow Trauma To Which Nerve
Apr 02, 2025
-
Another Term For The All Channel Communication Network Is
Apr 02, 2025
-
The Controllable Variance Is So Called Because It
Apr 02, 2025
-
The Graph Of The Relation S Is Shown Below
Apr 02, 2025
-
Which One Of The Following Is A Working Capital Decision
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about The Radical Below Can Be Stabilized By Resonance. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
