The Membrane Attack Stage Of The Complement Cascade Involves
Holbox
Mar 15, 2025 · 7 min read
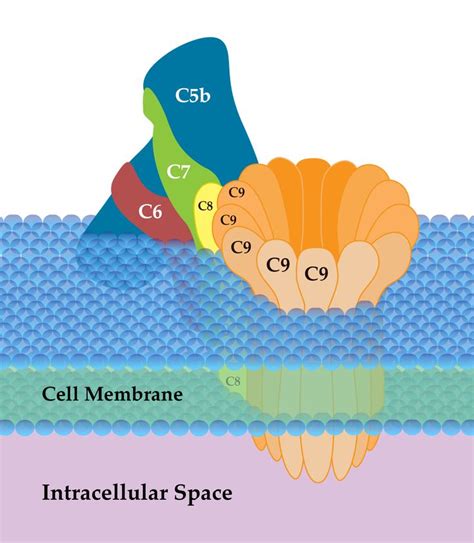
Table of Contents
The Membrane Attack Stage of the Complement Cascade: A Deep Dive
The complement system, a crucial part of the innate immune system, plays a vital role in defending against pathogens. This intricate network of proteins works in a cascade, amplifying the initial immune response and leading to the elimination of threats. One of the most impactful stages of this cascade is the membrane attack complex (MAC) formation, also known as the terminal complement pathway. This article will delve into the intricate details of the membrane attack stage, exploring its mechanisms, regulation, and significance in host defense.
Understanding the Complement Cascade: A Precursor to MAC Formation
Before diving into the specifics of MAC formation, it's crucial to understand the broader context of the complement cascade. This cascade is triggered by three distinct pathways:
- Classical Pathway: Activated by antibody-antigen complexes bound to pathogens.
- Alternative Pathway: Triggered spontaneously on pathogen surfaces, independent of antibodies.
- Lectin Pathway: Initiated by mannose-binding lectin (MBL) binding to carbohydrates on pathogen surfaces.
Regardless of the initiating pathway, all three converge at a central point – the activation of C3 convertase. This enzyme cleaves C3 into C3a and C3b. C3b is a pivotal component, crucial for both opsonization (enhancing phagocytosis) and the formation of the MAC.
The Formation of the Membrane Attack Complex (MAC)
The MAC, the culmination of the terminal complement pathway, is a cylindrical pore-forming structure that directly lyses target cells. Its formation is a complex process involving several key complement proteins:
1. C5 Convertase Formation: The Gateway to MAC
C3b, the product of C3 convertase, plays a crucial role in forming the C5 convertase. The exact composition of C5 convertase varies slightly depending on the initiating pathway:
- Classical and Lectin Pathways: C4b2a3b
- Alternative Pathway: C3bBb3b
C5 convertase, irrespective of its composition, catalyzes the cleavage of C5 into C5a and C5b. C5a is a potent anaphylatoxin, contributing to inflammation, while C5b initiates the assembly of the MAC.
2. The Assembly of the MAC: A Step-by-Step Process
The assembly of the MAC is a carefully orchestrated process, involving the sequential binding of several complement proteins:
- C5b Binding: C5b, initially unstable, quickly binds to C6. This interaction stabilizes C5b and facilitates further assembly.
- C7 Binding: The C5b6 complex then recruits C7, leading to a conformational change that exposes a hydrophobic region. This region allows the complex to insert itself into the target cell membrane.
- C8 Binding: C8, composed of three subunits (α, β, and γ), binds to the C5b67 complex. The C8γ subunit inserts into the target cell membrane, anchoring the complex.
- C9 Polymerization: Finally, multiple molecules of C9 bind to the C5b678 complex, forming a poly-C9 ring, the central pore of the MAC. This ring creates a transmembrane channel, disrupting the integrity of the target cell membrane.
This precise assembly ensures the efficient formation of a functional pore, capable of causing cell lysis.
The Role of MAC in Host Defense: A Multifaceted Weapon
The MAC's primary function is the lysis of target cells, including bacteria, viruses, and even some host cells (in cases of dysregulation). However, its contribution to host defense goes beyond simple cell death. It contributes to:
- Direct Cell Lysis: The most prominent function of the MAC is its ability to create pores in the cell membrane, leading to osmotic lysis and cell death. This is particularly effective against Gram-negative bacteria, which have a thinner peptidoglycan layer compared to Gram-positive bacteria.
- Inflammation Amplification: The MAC can indirectly contribute to inflammation through the release of various inflammatory mediators. For example, the formation of the MAC can trigger the release of intracellular contents from the lysed cell, further stimulating the inflammatory response.
- Enhancement of Phagocytosis: Although not a primary function, the MAC can enhance the process of phagocytosis (the engulfment of pathogens by immune cells). The presence of MAC on the pathogen surface can act as an "eat me" signal, attracting and facilitating phagocytosis by macrophages and neutrophils.
Regulation of the Complement Cascade: Preventing Unwanted Damage
The complement cascade, powerful as it is, requires tight regulation to prevent damage to host cells. Uncontrolled activation can lead to autoimmune diseases and tissue damage. Several mechanisms exist to regulate MAC formation:
- Decay Accelerating Factor (DAF): DAF is a cell-surface protein that disrupts C3 convertase, preventing its continued activity and limiting the formation of C5 convertase and subsequently the MAC.
- CD59 (Protectin): This protein binds to the C5b678 complex, preventing C9 polymerization and the formation of the complete MAC pore. This is particularly crucial for protecting host cells from self-destruction.
- Factor I: This serine protease cleaves C3b and C4b, thereby inactivating them and preventing the formation of both C3 and C5 convertases.
- Factor H: This protein acts as a cofactor for Factor I, enhancing its ability to cleave C3b. It also competes with C3b for binding to host cell surfaces, preventing the formation of the alternative pathway C3 convertase.
- Sialic Acid: The presence of sialic acid on the surface of host cells helps to prevent the inappropriate activation of the complement cascade. This happens because the alternative pathway is less likely to initiate on surfaces with sialic acid.
These regulatory mechanisms are essential for maintaining a delicate balance, ensuring the complement system effectively combats pathogens without causing harm to the host.
Clinical Significance of MAC Dysregulation: A Range of Diseases
Dysregulation of the complement cascade, particularly the terminal pathway and MAC formation, is implicated in a range of diseases:
- Paroxysmal Nocturnal Hemoglobinuria (PNH): This rare blood disorder results from a deficiency in GPI-anchored proteins, including DAF and CD59. This lack of regulation leads to excessive complement activation and destruction of red blood cells, resulting in anemia and hemoglobinuria.
- Atypical Hemolytic Uremic Syndrome (aHUS): This is a serious condition characterized by thrombotic microangiopathy, leading to kidney failure, thrombocytopenia (low platelet count), and hemolytic anemia. Genetic mutations affecting complement regulatory proteins, such as Factor H, Factor I, and membrane cofactor protein (MCP), are often responsible for aHUS.
- Age-Related Macular Degeneration (AMD): While the exact mechanisms are still being investigated, complement activation plays a significant role in the pathogenesis of AMD, a leading cause of vision loss in the elderly. Abnormal complement activation in the retina can cause damage to the photoreceptors and retinal pigment epithelium.
- Systemic Lupus Erythematosus (SLE): In SLE, an autoimmune disease, the complement system is frequently activated, contributing to tissue damage and inflammation. Reduced complement levels are often observed in SLE patients.
Future Research and Therapeutic Implications: Targeting the MAC
The intricate nature of the complement system and its significant role in both health and disease make it a promising target for therapeutic interventions. Future research could focus on:
- Developing more specific inhibitors: Current complement inhibitors often lack the specificity needed to target only the unwanted activation while preserving the beneficial effects of the system. Future research should focus on designing inhibitors that specifically target the MAC formation without affecting other important complement functions.
- Identifying novel therapeutic targets: Further investigation into the regulatory mechanisms of the complement system might reveal new therapeutic targets for diseases where complement dysregulation is a key factor.
- Personalized medicine approaches: Genetic variations influencing complement activity are substantial. Personalized medicine approaches, tailoring therapies based on individual genetic profiles, could significantly improve treatment outcomes.
- Understanding the role of the MAC in specific diseases: A deeper understanding of the specific role of the MAC in various diseases can guide the development of targeted therapies. For example, selective inhibition of the MAC formation might be particularly beneficial in diseases like PNH and aHUS, where uncontrolled complement activation plays a central role.
The membrane attack stage of the complement cascade, culminating in the formation of the MAC, represents a powerful arm of the innate immune system. Understanding the intricacies of MAC formation, regulation, and clinical significance is crucial for developing novel therapeutic strategies to treat a wide spectrum of diseases characterized by complement dysregulation. Continued research into this complex system holds immense promise for improving human health.
Latest Posts
Latest Posts
-
What Windows Application Stores Events Logged By The Operating System
Mar 15, 2025
-
Affirmative Action Programs Have Generated Many Opportunities For And
Mar 15, 2025
-
A Flexible Budget Performance Report Compares
Mar 15, 2025
-
During The Breakfast Rush An Angry Customer
Mar 15, 2025
-
Is The Characteristic Of The Individuals Within The Population
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about The Membrane Attack Stage Of The Complement Cascade Involves . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
