The Correct Name Of The Compound Ncl3 Is
Holbox
Mar 19, 2025 · 5 min read
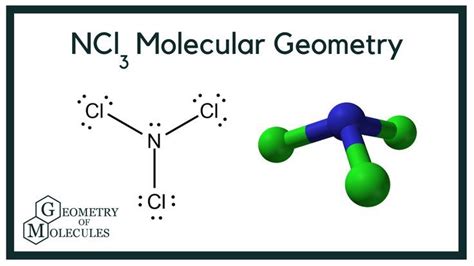
Table of Contents
The Correct Name of the Compound NCl3 Is: Nitrogen Trichloride – A Deep Dive into Nomenclature and Properties
The seemingly simple question, "What is the correct name of the compound NCl3?" opens a door to a fascinating exploration of chemical nomenclature, bonding, and the properties of this intriguing substance. While the answer itself is straightforward – nitrogen trichloride – understanding why this is the correct name requires a delve into the principles of chemical naming conventions and the specific characteristics of this compound. This article will not only provide the definitive answer but also explore the compound's structure, reactivity, and historical context, enriching your understanding of inorganic chemistry.
Understanding Chemical Nomenclature: A Foundation
Before diving into the specifics of NCl3, let's establish the fundamental rules of inorganic chemical nomenclature. These rules, developed by the International Union of Pure and Applied Chemistry (IUPAC), ensure consistent and unambiguous naming of chemical compounds globally. The system is based on several key principles:
1. Cation First, Anion Second:
In binary compounds (compounds composed of two elements), the cation (positively charged ion) is named first, followed by the anion (negatively charged ion). This principle applies to both ionic and covalent compounds, although the naming conventions differ slightly.
2. Roman Numerals for Variable Oxidation States:
Some elements can exhibit multiple oxidation states (the charge of an ion). For transition metals and some post-transition metals, Roman numerals in parentheses are used to indicate the oxidation state. For example, FeCl2 is iron(II) chloride, and FeCl3 is iron(III) chloride.
3. Prefixes for Covalent Compounds:
Covalent compounds, where atoms share electrons rather than transferring them, utilize prefixes to indicate the number of atoms of each element present. These prefixes include:
- Mono- (1)
- Di- (2)
- Tri- (3)
- Tetra- (4)
- Penta- (5)
- Hexa- (6)
- Hepta- (7)
- Octa- (8)
- Nona- (9)
- Deca- (10)
The prefix "mono-" is often omitted for the first element unless it is necessary for clarity.
Naming NCl3: Applying the Rules
Now, let's apply these rules to NCl3. Nitrogen (N) is less electronegative than chlorine (Cl), meaning it will have a positive oxidation state, making it the cation. Chlorine will be the anion. Nitrogen typically exhibits a -3, +1, +2, +3, +4, or +5 oxidation state in its compounds. In NCl3, nitrogen is in the +3 oxidation state and chlorine is -1. Considering the covalent nature of this compound due to the relatively small electronegativity difference between nitrogen and chlorine and applying the prefixes, we arrive at the correct name: nitrogen trichloride.
The Structure and Bonding of NCl3: A Closer Look
The molecule NCl3 is a trigonal pyramidal shape with nitrogen at the apex and three chlorine atoms forming the base. This geometry is predicted by the valence shell electron pair repulsion (VSEPR) theory, which states that electron pairs around a central atom will arrange themselves to minimize repulsion. The nitrogen atom has one lone pair of electrons and three bonding pairs, resulting in the pyramidal structure. The bonds are covalent, formed by the sharing of electrons between nitrogen and chlorine atoms.
Polarity and Intermolecular Forces:
The NCl3 molecule is polar due to the difference in electronegativity between nitrogen and chlorine and the asymmetrical arrangement of atoms. This polarity leads to dipole-dipole interactions between molecules, contributing to its physical properties like boiling point.
Properties of Nitrogen Trichloride: Reactivity and Hazards
Nitrogen trichloride is a highly reactive and dangerous compound. Its instability stems from the relatively weak N-Cl bonds. It is a volatile liquid at room temperature, and it is extremely sensitive to light and heat, readily decomposing explosively. Even minor disturbances can trigger its decomposition, producing toxic chlorine gas and releasing significant energy. It is imperative to handle this compound with extreme caution in a well-ventilated area, preferably under strictly controlled laboratory conditions and with specialized safety equipment.
Historical Context and Applications:
Although highly dangerous, NCl3 has a place in chemical history. It was initially discovered and studied in the 19th century. Despite its hazardous nature, it has found extremely limited applications in specific niche industrial processes, predominantly in the past. The high risks associated with its production and use have led to its near-total replacement by safer alternatives.
Distinguishing NCl3 from Other Nitrogen Chlorides:
It's important to note that there are other nitrogen chlorides, and differentiating them from NCl3 is critical to avoid confusion and ensure safety. These include:
- Nitrogen dichloride (NCl2): This is a highly unstable and reactive radical.
- Nitrogen pentachloride (NCl5): This compound is theoretically possible but has never been successfully synthesized due to steric hindrance and the high oxidation state of nitrogen involved. The relative sizes of nitrogen and chlorine atoms prevent such a configuration.
The unique chemical formula and the resulting distinct properties clearly separate NCl3 from these other nitrogen chlorides.
Safety Precautions: Handling and Storage of NCl3:
Given its extreme reactivity and danger, working with NCl3 demands meticulous adherence to safety protocols. These include:
- Specialized training: Personnel handling NCl3 should undergo comprehensive safety training.
- Protective equipment: This should include respirators with appropriate filters, chemical-resistant gloves, lab coats, and eye protection.
- Controlled environment: Work should only be undertaken in a well-ventilated fume hood.
- Controlled temperature and light: Storage and handling should take place at low temperatures and away from direct light.
- Emergency procedures: Well-defined emergency plans and proper first-aid measures must be readily available.
Further Research and Exploration:
The chemistry of NCl3 remains a subject of ongoing research. Studies continue to investigate its decomposition pathways, the mechanisms of its reactivity, and the potential for safer, albeit limited, applications.
Conclusion:
The correct name for the compound NCl3 is undeniably nitrogen trichloride. However, this simple answer encapsulates a wealth of knowledge concerning chemical nomenclature, bonding theory, molecular structure, and the crucial aspects of chemical safety. Understanding the properties and hazards of NCl3 is paramount for anyone working in chemistry, highlighting the importance of meticulous attention to detail and robust safety procedures when dealing with such hazardous substances. This comprehensive exploration serves not only to provide the correct name but also to build a richer understanding of the broader chemical principles involved and emphasize the critical role of safety in chemical practice.
Latest Posts
Latest Posts
-
Budget Compare Actual Results To Budgeted Results
Mar 19, 2025
-
Find As A Function Of If
Mar 19, 2025
-
Where Is Velocity Highest In A River
Mar 19, 2025
-
Cups And Glasses Are Taking Too Long To Air Dry
Mar 19, 2025
-
Air Pollution Is Accurately Described By Which Statement
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about The Correct Name Of The Compound Ncl3 Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
