Select The Nmr Spectrum That Corresponds Best To P-anisidine
Holbox
Mar 15, 2025 · 5 min read
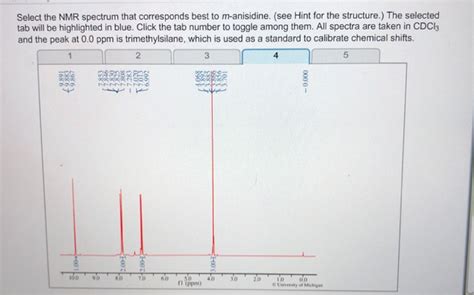
Table of Contents
Selecting the NMR Spectrum that Corresponds Best to p-Anisidine: A Comprehensive Guide
p-Anisidine, also known as 4-methoxyaniline, is a simple aromatic compound with a distinct NMR signature. Successfully identifying its spectrum requires understanding the principles of nuclear magnetic resonance (NMR) spectroscopy and the chemical environment of the protons and carbons within the molecule. This article will delve into the expected NMR spectra (both ¹H and ¹³C NMR) for p-anisidine, providing a detailed analysis to help you confidently select the correct spectrum from a given set.
Understanding the Structure of p-Anisidine
Before we dive into spectral analysis, let's examine the structure of p-anisidine. The molecule consists of a benzene ring substituted with an amino group (-NH₂) at the para position (position 4) and a methoxy group (-OCH₃) at position 1. This substitution pattern significantly influences the chemical shifts observed in the NMR spectra.
- Para Substitution: The para arrangement of the amino and methoxy groups creates a symmetrical molecule, leading to certain simplifications in the NMR spectra.
- Amino Group (-NH₂): This group is electron-donating, affecting the chemical shifts of the aromatic protons. It also exhibits its own characteristic signal in the ¹H NMR spectrum.
- Methoxy Group (-OCH₃): This group is also electron-donating, but its effect is somewhat different from the amino group. It will have a distinct signal in both ¹H and ¹³C NMR.
- Aromatic Protons: The protons on the benzene ring experience different chemical environments due to the substituents, leading to distinct chemical shifts.
Interpreting the ¹H NMR Spectrum of p-Anisidine
The ¹H NMR spectrum provides information about the different types of protons in the molecule and their chemical environments. For p-anisidine, we expect to see several distinct signals:
Aromatic Protons:
- Two sets of doublets: Due to the para substitution, the aromatic protons will appear as two doublets (AA'BB' system) integrating to two protons each. The coupling constant (J) will be relatively small (around 8-9 Hz). The exact chemical shift will depend on the solvent used, but they typically appear in the range of 6.5-7.5 ppm. The protons ortho to the amino group will appear more upfield due to the electron-donating effect of the amino group. The protons ortho to the methoxy group will be further downfield.
Amino Protons:
- Broad singlet: The two amino protons (-NH₂) usually appear as a broad singlet (or sometimes a broad multiplet) in the range of 3-5 ppm. The broadness is due to hydrogen bonding and exchange with the solvent. This signal can often be identified by its characteristic broad shape and integration of two protons. The exact chemical shift and appearance will vary based on the solvent and concentration.
Methoxy Protons:
- Singlet: The three protons of the methoxy group (-OCH₃) will appear as a sharp singlet integrating to three protons, typically in the range of 3.5-4.0 ppm. The electron-donating nature of both the amino and methoxy groups influence the chemical shift, but these protons are generally deshielded compared to methoxy groups on less electron-rich benzene rings.
Interpreting the ¹³C NMR Spectrum of p-Anisidine
The ¹³C NMR spectrum provides information about the different types of carbon atoms in the molecule. For p-anisidine, we expect to see six distinct signals:
Aromatic Carbons:
- Four distinct signals: The six carbon atoms of the benzene ring will generate four distinct signals due to symmetry. The carbons bearing the substituents (C1 and C4) will have different chemical shifts compared to the other aromatic carbons (C2, C3, C2', C3'). The exact chemical shift will depend on the substituents and their electronic effects. Generally, carbons directly bonded to electron-donating groups show upfield shifts, while those further away are less affected.
Methoxy Carbon:
- One signal: The carbon atom of the methoxy group (-OCH₃) will give a distinct signal, usually in the range of 50-60 ppm. Its exact position is influenced by the neighboring aromatic ring.
Identifying the Correct Spectrum: A Step-by-Step Approach
When presented with a selection of NMR spectra, use the following steps to identify the one corresponding to p-anisidine:
-
Check the Number of Signals: Both ¹H and ¹³C NMR spectra should show the expected number of signals based on the molecule's symmetry and the number of chemically distinct protons and carbons.
-
Analyze Chemical Shifts: Carefully examine the chemical shifts of each signal. Do they fall within the expected ranges discussed above? Pay close attention to the aromatic protons, which will show distinct chemical shifts due to the para substitution.
-
Integration: Verify that the integration values in the ¹H NMR spectrum are consistent with the number of protons in each group.
-
Coupling Constants: In the ¹H NMR spectrum, the coupling constant (J) between the aromatic protons should be characteristic of an aromatic system.
-
Compare to Known Spectra: If available, comparing the unknown spectrum to a known, reliable spectrum of p-anisidine is invaluable.
-
Consider Solvent Effects: Remember that solvent effects can slightly alter chemical shifts. If the solvent used in the unknown spectrum is known, this information can aid in the analysis.
Potential Pitfalls and Considerations
-
Solvent Peaks: Be aware of solvent peaks and other impurities that may appear in the spectra. These peaks must be distinguished from those belonging to p-anisidine.
-
Peak Overlapping: In some cases, peaks might overlap, making it challenging to determine the number of signals and integration values. Using advanced techniques such as 2D NMR can help to resolve this issue.
-
Sample Purity: The quality of the NMR spectrum is directly related to the purity of the p-anisidine sample. Impurities can lead to additional peaks, complicating spectral interpretation.
Conclusion
Identifying the NMR spectrum of p-anisidine requires a systematic and thorough approach. By understanding the molecule's structure, the principles of NMR spectroscopy, and the expected chemical shifts, you can confidently select the correct spectrum from a given set of options. Remembering to consider potential pitfalls such as solvent peaks and overlapping signals will further enhance your accuracy in the analysis. The combination of detailed spectral analysis and careful consideration of potential complications will ensure successful identification. This comprehensive guide serves as a robust resource for confidently assigning p-anisidine's NMR spectra.
Latest Posts
Latest Posts
-
A Nurse Is Preparing To Administer Esomeprazole 40 Mg
Mar 15, 2025
-
A Bank Reconciliation Should Be Prepared Periodically Because
Mar 15, 2025
-
What Are Functions Of Motor Movements In The Alimentary Canal
Mar 15, 2025
-
Who Must Inspect A Pfas And How Often
Mar 15, 2025
-
What If Rna Polymerase To Bind More Tightly Than Normal
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Select The Nmr Spectrum That Corresponds Best To P-anisidine . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
