What If Rna Polymerase To Bind More Tightly Than Normal
Holbox
Mar 15, 2025 · 5 min read
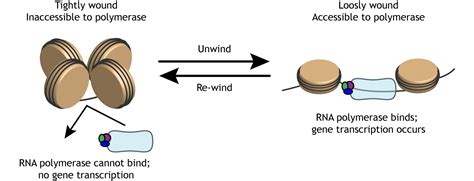
Table of Contents
What If RNA Polymerase Bound More Tightly Than Normal? Exploring the Ramifications of Enhanced Transcriptional Fidelity
RNA polymerase (RNAP), the molecular machine responsible for transcribing DNA into RNA, operates with remarkable precision. Its ability to bind to DNA promoters, initiate transcription, and accurately synthesize RNA molecules is crucial for cellular life. But what if this fundamental process were altered? What if RNAP bound to DNA more tightly than its normal, finely tuned affinity? This seemingly minor change could have profound and cascading effects across numerous cellular processes, potentially leading to both beneficial and detrimental consequences. This article explores the potential ramifications of such a scenario.
Altered Transcription Initiation: A Sticking Point?
The enhanced binding affinity of RNAP would significantly impact transcription initiation. Normally, RNAP's interaction with the promoter region is carefully regulated, allowing for precise control of gene expression. Increased binding strength could lead to several key changes:
1. Increased Transcriptional Frequency: A Flood of Transcripts?
A tighter grip on the promoter means RNAP would be less likely to dissociate before initiating transcription. This could result in a dramatic increase in the frequency of transcription initiation for a given gene. While seemingly beneficial at first glance – more protein production! – this could quickly overwhelm cellular machinery. The cell might be inundated with an excessive amount of mRNA transcripts, leading to:
- Ribosomal Bottlenecks: Ribosomes, responsible for translating mRNA into proteins, would be unable to keep pace with the deluge of transcripts. This could lead to stalled ribosomes, inefficient protein synthesis, and the accumulation of untranslated mRNA, potentially triggering cellular stress responses.
- Resource Depletion: The increased demand for RNA nucleotides and energy would strain cellular resources, potentially diverting energy from other essential processes.
- Protein Aggregation: Overproduction of proteins could lead to protein misfolding and aggregation, potentially causing cellular dysfunction and toxicity.
2. Reduced Promoter Selectivity: Transcriptional Chaos?
The tighter binding might also affect the specificity of promoter recognition. RNAP might bind non-specifically to DNA sequences that would normally be ignored, leading to indiscriminate transcription of non-coding regions and potentially harmful genes. This could result in:
- Production of Non-Functional Proteins: Transcription of non-coding sequences or genes with deleterious effects could lead to the synthesis of non-functional proteins, which might interfere with cellular processes or even be toxic.
- Increased Genomic Instability: Aberrant transcription could interfere with DNA replication and repair mechanisms, potentially increasing the risk of mutations and genomic instability.
- Disruption of Gene Regulation: The fine-tuned balance of gene expression, carefully controlled by various regulatory mechanisms, could be completely disrupted, leading to chaotic cellular behavior.
Altered Elongation and Termination: A Never-Ending Story?
The increased binding affinity wouldn't just affect initiation; it would also impact elongation and termination phases of transcription.
1. Impeded Elongation: Slowing Down the Transcriptional Train?
While a strong binding to the promoter might boost initiation, a similarly strong grip on the DNA during elongation could be problematic. The RNAP needs to move along the DNA template efficiently. Excessive binding might slow down or even stall the elongation process, resulting in:
- Incomplete Transcripts: Premature termination of transcription could generate truncated mRNA molecules, incapable of being translated into functional proteins.
- Formation of R-loops: The persistent binding of RNAP could lead to the formation of R-loops, which are structures formed by the hybridisation of nascent RNA with the DNA template strand. R-loops can interfere with DNA replication and repair, potentially leading to genomic instability and mutations.
2. Difficult Termination: A Transcriptional Deadlock?
The termination of transcription is another critical step that relies on the controlled release of RNAP from the DNA template. An abnormally strong interaction might prevent RNAP from disengaging properly, resulting in:
- Read-through Transcription: RNAP might continue transcribing past the normal termination signals, potentially leading to the production of abnormally long transcripts that could interfere with other cellular processes.
- Overlapping Transcription: The persistence of RNAP on the DNA template could interfere with the initiation of transcription on adjacent genes, creating a transcriptional conflict.
Cellular Responses to Enhanced RNAP Binding: A Fight for Survival?
The cell would likely mount several responses to counteract the effects of overly tight RNAP binding. These responses might include:
- Increased Expression of Transcriptional Regulators: The cell might attempt to increase the expression of proteins involved in regulating transcription initiation and termination, to try and restore some level of control over the process.
- Activation of Stress Response Pathways: The cellular stress caused by excessive transcription, protein aggregation, and resource depletion would trigger various stress response pathways, potentially leading to cellular senescence or apoptosis (programmed cell death).
- Mutations Affecting RNAP Structure: Over time, mutations in the RNAP gene itself might arise, potentially leading to the selection of RNAP variants with reduced binding affinity, restoring a more balanced transcriptional landscape.
Potential Beneficial Aspects: A Silver Lining?
While the predominantly negative consequences are apparent, there might be some limited beneficial aspects to increased RNAP binding under specific, tightly controlled conditions:
- Enhanced Fidelity of Transcription: A stronger interaction might, in theory, lead to increased accuracy during transcription, reducing the incidence of errors and mutations. This effect, however, would likely be minimal compared to the numerous detrimental consequences.
- Increased Expression of Crucial Genes: Under specific conditions, enhanced transcription of a critical gene might offer a survival advantage in a stressful environment. However, this benefit would be highly context-dependent and unlikely to outweigh the overall negative impact.
Conclusion: A Delicate Balance
The hypothetical scenario of RNA polymerase binding more tightly than normal highlights the delicate balance that maintains proper cellular function. The finely tuned affinity of RNAP for DNA is crucial for precise gene regulation and overall cellular homeostasis. A significant alteration in this affinity would likely have far-reaching and predominantly detrimental consequences, affecting transcription initiation, elongation, termination, and triggering various cellular stress responses. While limited beneficial aspects might exist under highly specific conditions, the overwhelming impact would likely be catastrophic for the cell, emphasizing the critical importance of maintaining the normal, finely tuned interactions within the transcriptional machinery. Further research into the specific mechanisms involved in RNAP binding and transcriptional regulation is crucial for a more complete understanding of this fundamental biological process.
Latest Posts
Latest Posts
-
In Which Situations Can Simplifying Jobs Be Most Beneficial
Mar 17, 2025
-
For The Hr Planning Process How Should Goals Be Determined
Mar 17, 2025
-
How Does A Shortcut Link To Another File
Mar 17, 2025
-
Cash Flows From Financing Activities Do Not Include
Mar 17, 2025
-
A Positive Return On Investment For Education Happens When
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What If Rna Polymerase To Bind More Tightly Than Normal . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
