Red Blood Cells Put In A Hypertonic Solution Will
Holbox
Mar 16, 2025 · 6 min read
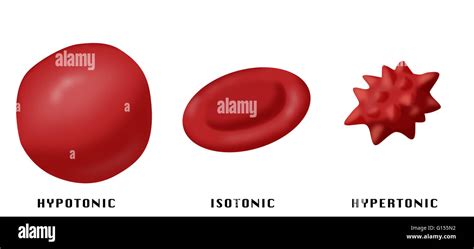
Table of Contents
Red Blood Cells in a Hypertonic Solution: A Deep Dive into Osmosis and Cell Function
Understanding how cells react to different environments is fundamental to biology. One crucial concept in this field is osmosis, the movement of water across a selectively permeable membrane from a region of high water concentration to a region of low water concentration. This movement is driven by the difference in water potential between the two regions. This article will delve into the specific effects of placing red blood cells (RBCs), also known as erythrocytes, into a hypertonic solution, exploring the underlying mechanisms and their physiological consequences.
What is a Hypertonic Solution?
Before discussing the effects on red blood cells, let's define a hypertonic solution. A hypertonic solution is one that has a higher solute concentration compared to another solution, in this case, the interior of the red blood cell. This higher solute concentration means there's a lower concentration of water outside the cell than inside. The key element here is the concentration gradient, the difference in solute concentration across the cell membrane. This gradient drives the movement of water.
Osmosis and Water Movement
The cell membrane of a red blood cell is selectively permeable, meaning it allows some substances to pass through while restricting others. Water molecules, being small and uncharged, can freely move across the membrane through specialized protein channels called aquaporins. However, larger molecules like proteins and sugars are largely unable to cross the membrane.
When a red blood cell is placed in a hypertonic solution, the concentration gradient causes water to move out of the cell. Water follows the principle of moving from an area of high water concentration (inside the cell) to an area of low water concentration (outside the cell) to try and equalize the concentration on both sides of the membrane. This process is driven by the natural tendency to achieve equilibrium.
The Effect on Red Blood Cells: Crenation
The net loss of water from the red blood cell in a hypertonic solution leads to a phenomenon known as crenation. Crenation refers to the shrinking of the cell, causing it to become wrinkled and spiky in appearance. The cell membrane buckles and invaginates as the cell's volume decreases significantly due to the outward movement of water. This dramatic change in cell shape affects its ability to perform its primary function: oxygen transport.
The Mechanism of Crenation
The process of crenation is a direct consequence of osmosis. The water potential inside the cell is higher than the water potential outside the cell in a hypertonic environment. This difference in water potential drives the osmotic flow of water out of the cell. As water leaves, the cell loses its turgor pressure, the pressure exerted by the cell contents against the cell wall (in plant cells) or cell membrane (in animal cells). This loss of turgor pressure causes the cell membrane to collapse inward, resulting in the characteristic crenated appearance.
Physiological Consequences of Crenation
Crenation significantly compromises the functionality of red blood cells. The changes in cell shape and volume affect several key aspects of their normal operation:
-
Reduced Oxygen Carrying Capacity: The altered shape of the crenated cell impairs its ability to efficiently deform and pass through narrow capillaries. This reduced flexibility hinders oxygen delivery to the body's tissues, leading to potential oxygen deprivation.
-
Impaired Membrane Integrity: The stress on the cell membrane during crenation can lead to membrane damage. This damage can cause leakage of intracellular components, further impacting the cell's function and potentially triggering an immune response.
-
Hemolysis and Cell Death: In severe cases of hypertonicity, the excessive water loss can lead to cell death. While crenation is a reversible process if the cell is returned to an isotonic environment, prolonged exposure to a hypertonic solution can result in irreversible damage and eventual lysis (cell rupture).
Comparing Hypertonic, Hypotonic, and Isotonic Solutions
Understanding the effects of a hypertonic solution requires comparing it to hypotonic and isotonic solutions:
-
Hypotonic Solution: A hypotonic solution has a lower solute concentration compared to the cell's interior. In this case, water moves into the red blood cell, causing it to swell and potentially lyse (burst). This is known as hemolysis.
-
Isotonic Solution: An isotonic solution has the same solute concentration as the cell's interior. In this case, there is no net movement of water across the membrane, and the cell maintains its normal shape and function.
The difference in osmotic pressure between the solution and the cell interior determines the direction and extent of water movement. This difference highlights the importance of maintaining an isotonic environment for optimal cell function.
Real-World Examples and Applications
The concept of hypertonic solutions and their effects on red blood cells has numerous applications in various fields, including:
-
Medicine: Intravenous fluids administered to patients must be isotonic to prevent hemolysis or crenation. Hypertonic saline solutions are sometimes used in specific medical situations, such as treating cerebral edema (brain swelling), where their ability to draw water out of swollen tissues is beneficial. However, the use of hypertonic solutions requires careful monitoring to prevent adverse effects.
-
Food Preservation: High concentrations of salt or sugar create hypertonic environments that inhibit the growth of microorganisms by drawing water out of bacterial cells, thus preventing spoilage. This is a common principle used in pickling, canning, and other food preservation techniques.
-
Laboratory Settings: Researchers utilize hypertonic solutions in various experiments to study cellular processes and responses to osmotic stress. These experiments can provide valuable insights into cell biology, membrane transport, and the mechanisms of cell damage.
Further Exploration: Factors Influencing Crenation
Several factors can influence the extent and rate of crenation in red blood cells exposed to a hypertonic solution:
-
Concentration of the Hypertonic Solution: The higher the solute concentration, the greater the osmotic gradient and the more water will move out of the cell, leading to more pronounced crenation.
-
Duration of Exposure: Prolonged exposure to a hypertonic solution results in more severe crenation than brief exposure.
-
Cell Age and Health: Older or damaged red blood cells may be more susceptible to crenation than healthy, younger cells. Their membranes might be less robust and less able to withstand the osmotic stress.
-
Temperature: Temperature can affect the permeability of the cell membrane. Higher temperatures might increase permeability, leading to faster water movement and more rapid crenation.
Conclusion: The Significance of Osmosis in Cellular Function
The effects of placing red blood cells in a hypertonic solution clearly demonstrate the crucial role of osmosis in maintaining cell structure and function. Crenation, the result of water loss from cells in a hypertonic environment, highlights the importance of maintaining osmotic balance. Understanding these principles is not only vital for basic biological knowledge but also has crucial implications for various applications in medicine, food science, and research. Further study into osmotic pressure and its effects on cells continues to provide valuable insights into the complex mechanisms that govern cellular life. The sensitivity of red blood cells to osmotic changes underscores the necessity for maintaining a controlled environment to ensure their proper functioning and the overall health of the organism.
Latest Posts
Latest Posts
-
All Of The Following Accurately Describe Lockout Tags Except
Mar 17, 2025
-
What Is The Narrowest Definition Of The Number 6
Mar 17, 2025
-
Strategic Implementation Is Thought To Be
Mar 17, 2025
-
Laker Company Reported The Following January
Mar 17, 2025
-
Quantitative Analysis Of Vinegar Via Titration
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Red Blood Cells Put In A Hypertonic Solution Will . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
