Rank The Structures In Order Of Decreasing Electrophile Strength
Holbox
Mar 26, 2025 · 6 min read
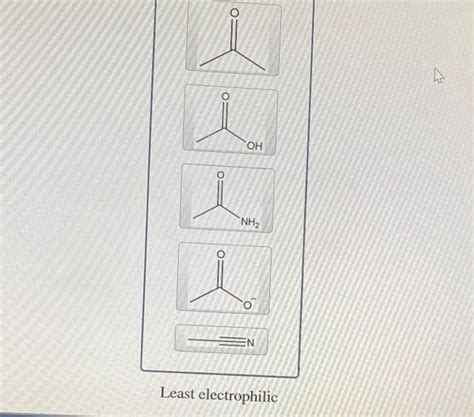
Table of Contents
- Rank The Structures In Order Of Decreasing Electrophile Strength
- Table of Contents
- Ranking Electrophiles: A Comprehensive Guide to Decreasing Electrophile Strength
- Factors Influencing Electrophile Strength
- 1. Positive Charge Density:
- 2. Electronegativity of Attached Atoms:
- 3. Resonance Stabilization:
- 4. Steric Hindrance:
- 5. Hybridization:
- Ranking Electrophiles: From Strongest to Weakest
- Influence of Reaction Conditions
- Examples and Further Elaboration
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Ranking Electrophiles: A Comprehensive Guide to Decreasing Electrophile Strength
Electrophiles, meaning "electron-loving," are crucial reagents in organic chemistry. They participate in electrophilic reactions, where they accept electron pairs from nucleophiles (electron-rich species). Understanding the relative strengths of electrophiles is fundamental to predicting reaction outcomes and designing efficient synthetic strategies. This article will comprehensively rank various electrophiles in order of decreasing strength, explaining the underlying principles and factors influencing their electrophilicity.
Factors Influencing Electrophile Strength
Several factors determine an electrophile's strength. These include:
1. Positive Charge Density:
A higher positive charge density directly translates to a stronger electrophile. Species with full positive charges are generally stronger than those with partial positive charges.
2. Electronegativity of Attached Atoms:
Electronegative atoms (e.g., oxygen, fluorine) withdraw electron density from the electrophilic center, increasing its positive charge and electrophilicity. Conversely, electron-donating groups decrease electrophilicity.
3. Resonance Stabilization:
If the electrophile can be stabilized through resonance, its electrophilicity will be reduced. Resonance delocalizes the positive charge, making it less reactive.
4. Steric Hindrance:
Bulky groups surrounding the electrophilic center can hinder nucleophilic attack, reducing the electrophile's reactivity.
5. Hybridization:
The hybridization of the electrophilic carbon atom influences its electrophilicity. sp-hybridized carbons are more electronegative than sp<sup>2</sup> or sp<sup>3</sup> hybridized carbons, making them stronger electrophiles.
Ranking Electrophiles: From Strongest to Weakest
The following ranking provides a general guideline. The relative strengths can vary depending on the specific reaction conditions and the nucleophile involved.
Tier 1: Strong Electrophiles
-
Carbocations: These species possess a full positive charge on a carbon atom. Tertiary carbocations (R<sub>3</sub>C<sup>+</sup>) are generally more stable (and therefore less reactive) than secondary (R<sub>2</sub>CH<sup>+</sup>) and primary (RCH<sub>2</sub><sup>+</sup>) carbocations due to inductive and hyperconjugative effects. Methyl carbocations (CH<sub>3</sub><sup>+</sup>) are the least stable and most reactive.
-
Acyl halides (e.g., RCOCl): The carbonyl carbon carries a partial positive charge due to the electronegativity of the oxygen and the halide. This makes them highly reactive towards nucleophiles.
-
Acid anhydrides: Similar to acyl halides, the carbonyl carbons in acid anhydrides bear a partial positive charge, making them strong electrophiles.
Tier 2: Moderate Electropholes
-
Aldehydes and Ketones: The carbonyl carbon in aldehydes and ketones is electrophilic due to the polar nature of the carbonyl group. However, they are less reactive than acyl halides or acid anhydrides because the positive charge is less pronounced. Steric hindrance around the carbonyl group can further reduce reactivity.
-
Alkyl halides (e.g., R-X where X = Br, Cl, I): The carbon atom bonded to the halogen carries a partial positive charge due to the electronegativity of the halogen. Reactivity generally follows the order: R-I < R-Br < R-Cl. Tertiary alkyl halides are more reactive than secondary and primary due to steric factors.
-
Epoxides: The strained three-membered ring in epoxides makes the carbon atoms electrophilic. Nucleophilic attack opens the ring, relieving the ring strain.
Tier 3: Weak Electrophiles
-
Imines and Enamines: These contain a carbon-nitrogen double bond with a partial positive charge on the carbon. However, the resonance stabilization of the nitrogen lone pair reduces their electrophilicity significantly.
-
Michael acceptors (α,β-unsaturated carbonyl compounds): The β-carbon in these compounds is electrophilic, but the conjugation with the carbonyl group delocalizes the positive charge, decreasing its reactivity.
-
Carbon dioxide (CO<sub>2</sub>): While it has a formal charge of zero, the carbon atom in CO<sub>2</sub> is electrophilic due to the presence of two electronegative oxygen atoms. However, its electrophilicity is relatively weak.
Tier 4: Very Weak Electrophiles
-
Arenes (benzene and its derivatives): Arenes undergo electrophilic aromatic substitution, but the aromatic ring is relatively resistant to electrophilic attack due to its resonance stabilization. Stronger electrophiles and specific reaction conditions are required for these reactions to proceed.
-
Alkenes and Alkynes: These compounds have electron-rich pi bonds that can act as nucleophiles. They can participate in electrophilic addition reactions, but they are generally considered weak electrophiles.
Influence of Reaction Conditions
The relative strengths of electrophiles can be significantly influenced by reaction conditions:
-
Solvent: Polar solvents can stabilize carbocations, making them less reactive. A non-polar solvent, conversely, can enhance their electrophilicity.
-
Catalyst: Lewis acids can activate electrophiles by coordinating to the electron-rich site and further increasing the positive charge density.
-
Temperature: Higher temperatures generally increase the rate of electrophilic reactions.
Examples and Further Elaboration
Let's delve into some specific examples to illustrate the concepts discussed:
Comparing Acyl Chloride and Aldehyde:
Acyl chlorides (RCOCl) are stronger electrophiles than aldehydes (RCHO). The chlorine atom is significantly more electronegative than the oxygen atom in an aldehyde, leading to a greater partial positive charge on the carbonyl carbon of the acyl chloride. This makes it a more reactive electrophile.
Comparing Tertiary and Primary Carbocations:
A tertiary carbocation (R<sub>3</sub>C<sup>+</sup>) is less reactive (weaker electrophile) than a primary carbocation (RCH<sub>2</sub><sup>+</sup>). The alkyl groups in a tertiary carbocation provide greater electron donation through inductive and hyperconjugative effects, stabilizing the positive charge. A primary carbocation, lacking this stabilization, is a much stronger electrophile and thus more reactive.
Resonance Effects in Imines:
Imines (R<sub>2</sub>C=NR) have a partial positive charge on the carbon. However, the nitrogen lone pair can participate in resonance, delocalizing the positive charge and thus reducing the electrophilicity of the carbon. This contrasts with carbocations where no such resonance stabilization is possible.
Steric Effects in Ketones:
Ketones with bulky substituents are less reactive as electrophiles compared to ketones with smaller substituents. The steric bulk hinders the approach of the nucleophile, reducing the rate of reaction.
The Role of Lewis Acids:
Lewis acids, such as aluminum chloride (AlCl<sub>3</sub>), are frequently used in electrophilic aromatic substitution reactions. They coordinate to the electrophile (e.g., an acyl halide or alkyl halide), increasing its electrophilicity and making it a more potent electrophile for reaction with the aromatic ring.
Conclusion
Understanding the relative strengths of electrophiles is paramount in organic chemistry. Factors like positive charge density, electronegativity, resonance stabilization, steric hindrance, and hybridization all contribute to an electrophile's reactivity. While a clear-cut ranking is challenging due to context-dependent reactivity, the tiered approach outlined above, combined with an understanding of the influencing factors, provides a strong framework for predicting and manipulating the outcome of electrophilic reactions. Remember that the strength of an electrophile is always relative to the nucleophile involved and the reaction conditions. This article provides a foundation for deeper exploration into the fascinating world of electrophilic reactions.
Latest Posts
Latest Posts
-
Which Of The Following Best Describes The Sympathetic Division
Mar 30, 2025
-
When A Company Receives An Interest Bearing Note Receivable It Will
Mar 30, 2025
-
Classify Each Substituent As Electron Donating Or Electron Withdrawing
Mar 30, 2025
-
Levine Company Uses The Perpetual Inventory System
Mar 30, 2025
-
The Balance In Discount On Bonds Payable
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Rank The Structures In Order Of Decreasing Electrophile Strength . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
