Classify Each Substituent As Electron Donating Or Electron Withdrawing
Holbox
Mar 30, 2025 · 6 min read
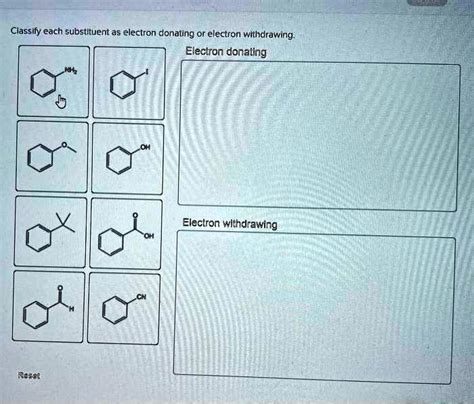
Table of Contents
- Classify Each Substituent As Electron Donating Or Electron Withdrawing
- Table of Contents
- Classify Each Substituent as Electron Donating or Electron Withdrawing
- Understanding Electron Donation and Withdrawal
- Inductive Effects
- Resonance Effects
- Classifying Substituents: A Practical Guide
- Strong Electron Withdrawing Groups (EWGs)
- Weak Electron Withdrawing Groups (EWGs)
- Strong Electron Donating Groups (EDGs)
- Weak Electron Donating Groups (EDGs)
- Predicting Reactivity: The Impact of Substituents
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Classify Each Substituent as Electron Donating or Electron Withdrawing
Understanding the electronic nature of substituents is fundamental to organic chemistry. Substituents, atoms or groups of atoms attached to a parent molecule, significantly influence the reactivity and properties of the parent compound. This influence stems from their ability to either donate or withdraw electron density. Accurately classifying substituents as electron donating or electron withdrawing is crucial for predicting reaction mechanisms, understanding spectroscopic data, and designing new molecules with desired properties. This article delves deep into this critical concept, exploring various factors that determine a substituent's electronic effect and providing a comprehensive guide for classification.
Understanding Electron Donation and Withdrawal
Before diving into specific examples, it's essential to understand the mechanisms behind electron donation and withdrawal. These effects primarily operate through two mechanisms: inductive effects and resonance effects.
Inductive Effects
Inductive effects are a result of the electronegativity difference between atoms within a molecule. Electronegativity refers to an atom's ability to attract electrons within a covalent bond. A more electronegative atom pulls electron density towards itself, creating a polar bond.
-
Electron Withdrawing Groups (EWGs): Groups containing highly electronegative atoms (e.g., halogens like F, Cl, Br, I; oxygen in –OH, –OR, –COOH; nitrogen in –NO₂, –CN) exert an electron-withdrawing inductive effect. They pull electron density away from the parent molecule, making the molecule more electron-deficient.
-
Electron Donating Groups (EDGs): Groups containing less electronegative atoms than carbon (e.g., alkyl groups like –CH₃, –C₂H₅) exert an electron-donating inductive effect. They push electron density towards the parent molecule, making the molecule more electron-rich.
The strength of the inductive effect diminishes with distance from the substituent. The closer the substituent is to the reactive center, the stronger its influence.
Resonance Effects
Resonance effects involve the delocalization of electrons through pi (π) bonds or lone pairs. This effect is particularly prominent in conjugated systems where alternating single and double bonds are present.
-
Electron Withdrawing Groups (EWGs): Groups with electron-withdrawing resonance effects contain atoms with empty orbitals that can accept electron density from the pi system. Examples include –NO₂, –CN, –CHO, and carbonyl groups (–C=O). These groups stabilize negative charge and destabilize positive charge on the ring.
-
Electron Donating Groups (EDGs): Groups with electron-donating resonance effects have lone pairs of electrons that can be delocalized into the pi system. Examples include –OH, –OR, –NH₂, –NHR, –NR₂. These groups stabilize positive charge and destabilize negative charge on the ring.
Classifying Substituents: A Practical Guide
Classifying substituents requires considering both inductive and resonance effects. Sometimes, one effect is dominant, while in other cases, both effects play significant roles.
Strong Electron Withdrawing Groups (EWGs)
These groups strongly pull electron density away from the parent molecule, often overriding any potential electron-donating inductive effects.
-
–NO₂ (Nitro group): This group is a powerful EWG due to both its strong inductive effect and its ability to withdraw electrons through resonance. The presence of two highly electronegative oxygen atoms and the possibility of resonance stabilization with the nitrogen makes it a strong deactivator and meta-director in electrophilic aromatic substitution reactions.
-
–CN (Cyano group): Similar to the nitro group, the cyano group possesses both a strong inductive and resonance electron-withdrawing effect. The triple bond and electronegative nitrogen make it a strong EWG.
-
–COOH (Carboxylic acid group): The carboxyl group's electronegative oxygen atoms and the ability to withdraw electrons through resonance make it a strong EWG.
-
–SO₃H (Sulfonic acid group): The sulfonic acid group, with its highly electronegative sulfur and oxygen atoms, effectively withdraws electrons, both inductively and through resonance.
-
Halogens (–F, –Cl, –Br, –I): Halogens exhibit a complex effect. They are inductively electron-withdrawing due to their high electronegativity. However, they can also donate electrons through resonance due to their lone pairs. Generally, the inductive effect dominates, making them weak electron-withdrawing groups. The strength of the inductive effect decreases down the group (F > Cl > Br > I).
Weak Electron Withdrawing Groups (EWGs)
These groups have a relatively weaker electron-withdrawing effect compared to the strong EWGs.
-
–CHO (Formyl group): The carbonyl group in the formyl group withdraws electrons both inductively and through resonance. However, compared to –NO₂ or –CN, its effect is weaker.
-
–COR (Acyl group): Similar to the formyl group, acyl groups withdraw electrons, but their strength is moderate.
-
–COOR (Ester group): Esters withdraw electrons inductively and through resonance but to a lesser extent than –NO₂ or –CN.
Strong Electron Donating Groups (EDGs)
These groups effectively push electron density towards the parent molecule, significantly influencing its reactivity.
-
–OH (Hydroxyl group): The hydroxyl group donates electrons through resonance due to its lone pair of electrons on the oxygen atom. It also exhibits a weak electron-withdrawing inductive effect, but the resonance effect generally dominates.
-
–OR (Alkoxy group): Similar to the hydroxyl group, alkoxy groups donate electrons through resonance.
-
–NH₂ (Amino group): The amino group is a very strong EDG, primarily because of its two lone pairs of electrons, which can readily participate in resonance.
-
–NHR (Alkyl amino group): These groups are strong EDGs, though slightly weaker than the amino group due to the electron-withdrawing effect of the alkyl group.
-
–NR₂ (Dialkyl amino group): Similar to the alkyl amino groups, they donate electrons strongly, although the strength slightly decreases compared to the amino group as the alkyl groups partially offset the donating ability.
Weak Electron Donating Groups (EDGs)
These groups have a weaker electron-donating effect than the strong EDGs.
- Alkyl groups (–CH₃, –C₂H₅, etc.): These groups are weak EDGs due to their inductive effect. They push electron density towards the parent molecule, but this effect diminishes with distance.
Predicting Reactivity: The Impact of Substituents
The electron-donating or electron-withdrawing nature of substituents profoundly impacts a molecule's reactivity. For example, in electrophilic aromatic substitution, electron-donating groups activate the aromatic ring towards electrophilic attack, while electron-withdrawing groups deactivate it. This is because EDGs increase electron density on the ring, making it more susceptible to electrophilic attack. Conversely, EWGs decrease electron density, making the ring less reactive. The position of substitution (ortho, meta, or para) is also affected by the substituent's electronic properties.
Similarly, in nucleophilic substitution reactions, electron-withdrawing groups enhance the reactivity of the substrate by increasing the partial positive charge on the carbon atom undergoing substitution. Conversely, electron-donating groups decrease reactivity.
Conclusion
Accurately classifying substituents as electron donating or electron withdrawing is a fundamental skill in organic chemistry. By understanding both inductive and resonance effects and considering their relative strengths, one can effectively predict the reactivity and properties of various organic molecules. This knowledge is crucial for designing and synthesizing new molecules with specific desired functionalities and for interpreting spectroscopic data and reaction mechanisms. The ability to analyze substituent effects allows for a deeper understanding of the intricate relationship between structure and reactivity in the fascinating world of organic chemistry.
Latest Posts
Latest Posts
-
How Do Groups Make It Through Storming
Apr 02, 2025
-
Draw A Second Resonance Form For The Structure Shown Below
Apr 02, 2025
-
Which Of The Following Is True Regarding An Acknowledgments Section
Apr 02, 2025
-
Lehninger Principles Of Biochemistry 8th Edition
Apr 02, 2025
-
Label The Cladogram Of Terrestrial Vertebrates
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Classify Each Substituent As Electron Donating Or Electron Withdrawing . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
