Rank The Following Atoms According To Their Size
Holbox
Mar 16, 2025 · 5 min read
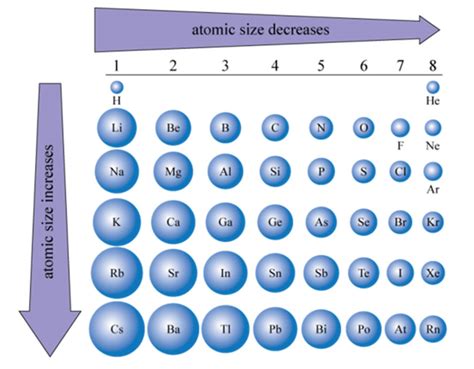
Table of Contents
Ranking Atoms by Size: A Comprehensive Guide
Determining the relative sizes of atoms is a fundamental concept in chemistry. While we can't directly "see" atoms, we can use various models and experimental data to understand and predict their sizes. This article will explore the factors that influence atomic size and provide a detailed ranking of several atoms, explaining the reasoning behind the order. We'll delve into the complexities of atomic radii, ionic radii, and the trends observed across the periodic table.
Understanding Atomic Size
Before we rank atoms, let's define what we mean by "size." In the atomic world, "size" is usually represented by atomic radius. This refers to half the distance between the nuclei of two identical atoms that are just touching each other. It's a measure of how far the atom's electron cloud extends. However, things get more complex when we consider ions (charged atoms). In those cases, we talk about ionic radius, which can be significantly different from the atomic radius, depending on whether the ion is a cation (positively charged) or an anion (negatively charged).
Factors Influencing Atomic Size
Several factors play a crucial role in determining an atom's size:
-
Principal Quantum Number (n): As we move down a group (column) in the periodic table, the value of 'n' increases. This means electrons are occupying higher energy levels, further from the nucleus, leading to a larger atomic radius. Think of it like adding more layers to an onion – the onion gets bigger.
-
Effective Nuclear Charge (Z<sub>eff</sub>): This is the net positive charge experienced by the outermost electrons. It's the difference between the number of protons in the nucleus (atomic number) and the shielding effect of inner electrons. A higher effective nuclear charge pulls the outermost electrons closer to the nucleus, resulting in a smaller atomic radius.
-
Shielding Effect: Inner electrons shield the outer electrons from the full positive charge of the nucleus. More inner electrons mean greater shielding, reducing the effective nuclear charge experienced by the outer electrons.
-
Electron-Electron Repulsion: Electrons repel each other. In atoms with many electrons, this repulsion can counteract the attractive force of the nucleus, slightly increasing the atomic radius.
Periodic Trends in Atomic Size
Understanding the periodic trends in atomic size is crucial for ranking atoms. Generally:
-
Atomic radius increases down a group: As we move down a group, the principal quantum number increases, placing outer electrons in higher energy levels further from the nucleus. The increased shielding effect also contributes.
-
Atomic radius decreases across a period (from left to right): As we move across a period, the number of protons in the nucleus increases, increasing the effective nuclear charge. This stronger pull on the electrons outweighs the increased electron-electron repulsion, resulting in a smaller atomic radius.
Ranking Atoms: A Practical Example
Let's rank the following atoms in order of increasing atomic size: Lithium (Li), Beryllium (Be), Boron (B), Carbon (C), Nitrogen (N), Oxygen (O), Fluorine (F), Neon (Ne).
These atoms all belong to the second period of the periodic table. Based on the periodic trends described above, we expect the atomic radius to decrease as we move from left to right. Therefore, the ranking should be:
Li < Be < B < C < N < O < F < Ne
Detailed Explanation
-
Lithium (Li): Lithium has the lowest effective nuclear charge among these atoms, resulting in the largest atomic radius.
-
Beryllium (Be), Boron (B), Carbon (C), Nitrogen (N), Oxygen (O), Fluorine (F), Neon (Ne): As we move across the period, the effective nuclear charge increases, pulling the outer electrons closer to the nucleus. Despite the increasing electron-electron repulsion, the increase in nuclear charge dominates, leading to a progressive decrease in atomic radius. Neon, being the farthest to the right (and thus having the highest effective nuclear charge), has the smallest atomic radius among these elements.
Comparing Atoms from Different Periods and Groups
Now let's consider a more complex comparison: Lithium (Li), Sodium (Na), Potassium (K), Fluorine (F), Chlorine (Cl), Bromine (Br). These atoms span multiple periods and groups, making the ranking slightly more challenging.
Here's the ranking in order of increasing atomic size:
F < Cl < Br < Li < Na < K
Detailed Explanation:
-
Halogens (F, Cl, Br): Fluorine, chlorine, and bromine are all halogens (Group 17). Atomic radius increases as we move down the group due to the addition of electron shells. Thus, F < Cl < Br.
-
Alkali Metals (Li, Na, K): Lithium, sodium, and potassium are alkali metals (Group 1). Again, atomic radius increases as we go down the group. Thus, Li < Na < K.
-
Comparing Groups: The halogens have significantly smaller atomic radii than the alkali metals. This is because the increased nuclear charge across a period has a stronger effect than the increased shielding and electron shell addition down a group. Even though potassium is in a higher principal energy level than Bromine, the nuclear charge of the halogens dominates.
Ionic Radii: A Further Consideration
The comparison becomes even more interesting when we consider ions. When an atom loses electrons to form a cation, its size decreases significantly because the remaining electrons are pulled more tightly towards the nucleus. Conversely, when an atom gains electrons to form an anion, its size increases because the increased electron-electron repulsion outweighs the increased nuclear attraction.
For instance, consider the ionic radii of Na⁺ and Cl⁻. Na⁺ is smaller than Na, while Cl⁻ is larger than Cl. The relative sizes depend on the number of electrons gained or lost and the resulting effective nuclear charge. Ranking ions requires considering these changes in addition to the factors affecting atomic radii.
Conclusion: A Dynamic Relationship
Ranking atoms by size isn't a simple task. It requires a deep understanding of the forces acting within an atom—the balance between nuclear attraction, electron-electron repulsion, and the shielding effect of inner electrons. The trends observed in the periodic table provide a framework for predicting relative atomic sizes. However, carefully considering factors like effective nuclear charge and the formation of ions is vital for accurately ranking atoms across different periods and groups. By understanding these principles, we can successfully predict and explain the relative sizes of a wide range of atoms and ions. Remember, this is a dynamic relationship constantly influenced by the intricate interplay of subatomic particles.
Latest Posts
Latest Posts
-
How Does A Shortcut Link To Another File
Mar 17, 2025
-
Cash Flows From Financing Activities Do Not Include
Mar 17, 2025
-
A Positive Return On Investment For Education Happens When
Mar 17, 2025
-
What Is The Value Of I
Mar 17, 2025
-
The Accounts In The Ledger Of Monroe Entertainment Co
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Rank The Following Atoms According To Their Size . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
