Predict The Product Of This Hofmann Elimination Reaction
Holbox
Mar 29, 2025 · 5 min read
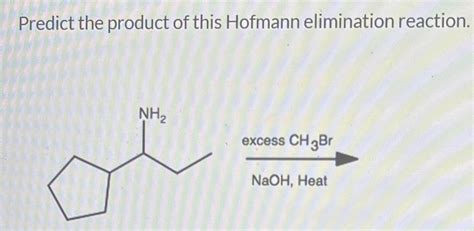
Table of Contents
- Predict The Product Of This Hofmann Elimination Reaction
- Table of Contents
- Predicting the Product of a Hofmann Elimination Reaction: A Comprehensive Guide
- Understanding the Hofmann Elimination Mechanism
- Step-by-Step Mechanism:
- Factors Influencing Regioselectivity: The Steric Hindrance Effect
- Visualizing the Steric Effect:
- Predicting the Product: A Step-by-Step Approach
- Considerations for Complex Cases
- Conclusion: Mastering Hofmann Elimination Prediction
- Latest Posts
- Latest Posts
- Related Post
Predicting the Product of a Hofmann Elimination Reaction: A Comprehensive Guide
The Hofmann elimination is a powerful reaction in organic chemistry used to synthesize alkenes from quaternary ammonium salts. Understanding the reaction mechanism and regioselectivity is crucial for predicting the major product. This comprehensive guide will delve into the intricacies of the Hofmann elimination, providing a step-by-step approach to predicting the product, along with examples and considerations for complex cases.
Understanding the Hofmann Elimination Mechanism
The Hofmann elimination involves the treatment of a quaternary ammonium salt with a strong base, typically silver oxide in water followed by heating. This process leads to the elimination of a tertiary amine and the formation of an alkene. Unlike the Saytzeff elimination, which favors the most substituted alkene, the Hofmann elimination exhibits a preference for the least substituted alkene. This regioselectivity is a defining characteristic and is crucial for predicting the product.
Step-by-Step Mechanism:
-
Formation of the Hydroxide Ion: The silver oxide (Ag₂O) reacts with water to produce silver hydroxide (AgOH), which then deprotonates water to form hydroxide ions (OH⁻).
-
Abstraction of a β-Hydrogen: The hydroxide ion acts as a strong base, abstracting a β-hydrogen (a hydrogen atom on the carbon adjacent to the nitrogen atom). This step is crucial for the regioselectivity observed in the Hofmann elimination.
-
Formation of a Transition State: A cyclic transition state forms, involving the hydroxide ion, the β-hydrogen, and the nitrogen atom. This transition state is crucial in dictating the stereochemistry and regioselectivity of the reaction.
-
Elimination of Trialkylamine: The nitrogen-carbon bond breaks, and a trialkylamine molecule is eliminated.
-
Formation of the Alkene: Simultaneously, a double bond (alkene) forms between the α-carbon (the carbon bonded to the nitrogen) and the β-carbon (the carbon from which the hydrogen was abstracted). This results in the formation of the least substituted alkene.
Factors Influencing Regioselectivity: The Steric Hindrance Effect
The preference for the least substituted alkene in the Hofmann elimination is primarily due to steric hindrance. The bulky trialkylammonium group significantly influences the transition state geometry. The base preferentially abstracts a β-hydrogen from the less substituted carbon because this leads to a less crowded transition state. This transition state has lower energy compared to the one that would lead to the more substituted alkene.
Visualizing the Steric Effect:
Imagine the bulky trialkylammonium group as a large, cumbersome substituent. Abstracting a hydrogen from a less substituted β-carbon allows for a more open transition state, minimizing steric interactions. Conversely, abstracting a hydrogen from a more substituted β-carbon would lead to a more crowded transition state, increasing the energy barrier and making it less favorable.
Predicting the Product: A Step-by-Step Approach
Predicting the product of a Hofmann elimination reaction involves identifying the β-hydrogens, considering steric hindrance, and determining the least substituted alkene formed. Let's break down the process with examples.
Example 1: Simple Hofmann Elimination
Consider the Hofmann elimination of N,N,N-trimethylbutan-1-ammonium hydroxide.
-
Identify the β-carbons: Locate the carbons adjacent to the nitrogen atom. In this case, there are two β-carbons.
-
Identify the β-hydrogens: Count the number of β-hydrogens on each β-carbon. One β-carbon has two hydrogens, and the other has one hydrogen.
-
Determine the least substituted alkene: Elimination of a β-hydrogen from the β-carbon with fewer hydrogens (one hydrogen in this case) leads to a less substituted alkene. This is the preferred product of the Hofmann elimination.
-
Draw the product: The major product will be but-1-ene.
Example 2: Hofmann Elimination with Multiple β-Hydrogens
Let's examine a more complex example, the Hofmann elimination of N,N,N-trimethyl-2-methylbutan-2-ammonium hydroxide.
-
Identify β-carbons: This molecule has three β-carbons.
-
Identify β-hydrogens: Two β-carbons have one hydrogen each, and one β-carbon has three hydrogens.
-
Determine the least substituted alkene: The base will preferentially abstract a hydrogen from the β-carbon with only one hydrogen. This leads to two possible products—2-methylbut-1-ene and 2-methylbut-2-ene—but the least substituted alkene (2-methylbut-1-ene) will be the major product due to less steric hindrance in its transition state.
-
Draw the product: The major product is 2-methylbut-1-ene.
Considerations for Complex Cases
In more complex molecules, several factors can influence the outcome of the Hofmann elimination. These factors include:
-
Multiple β-hydrogens: The presence of multiple β-hydrogens requires careful consideration of the steric hindrance around each β-carbon. The base will preferentially abstract a hydrogen from the least hindered position.
-
Stereochemistry: While the Hofmann elimination primarily focuses on regioselectivity, the stereochemistry of the starting material can influence the stereochemistry of the product. In cases with chiral centers, the elimination can proceed with either syn or anti elimination. The preference for either will depend on various factors including the steric bulk of substituents.
-
Competing Reactions: Other reactions, such as substitution reactions, can compete with the Hofmann elimination depending on the reaction conditions.
-
Aromatic Systems: Hofmann elimination is less effective for quaternary ammonium salts containing aryl groups directly attached to the nitrogen atom. The aromaticity stabilizes the system and prevents the elimination from occurring efficiently.
Conclusion: Mastering Hofmann Elimination Prediction
Predicting the product of a Hofmann elimination reaction requires a systematic approach that combines understanding the reaction mechanism, acknowledging the steric factors governing regioselectivity, and carefully analyzing the structure of the starting material. By systematically identifying β-hydrogens, assessing steric hindrance, and prioritizing the formation of the least substituted alkene, you can confidently predict the major product of even complex Hofmann elimination reactions. Remember to consider any additional influencing factors such as competing reactions or stereochemical implications for a complete and accurate prediction. This detailed analysis, coupled with practical exercises, will solidify your understanding and enable accurate predictions for various Hofmann elimination reactions you might encounter.
Latest Posts
Latest Posts
-
The Scores Of A Recent Test Taken By 1200
Apr 02, 2025
-
Which Of The Reactions Are Spontaneous Favorable
Apr 02, 2025
-
Two Systems Of Equations Are Given Below
Apr 02, 2025
-
Match The Hormone Abbreviations With Their Function
Apr 02, 2025
-
What Is Are The Product S Of The Following Reaction
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Predict The Product Of This Hofmann Elimination Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
