Predict The Major Product For The Reaction
Holbox
Mar 17, 2025 · 6 min read
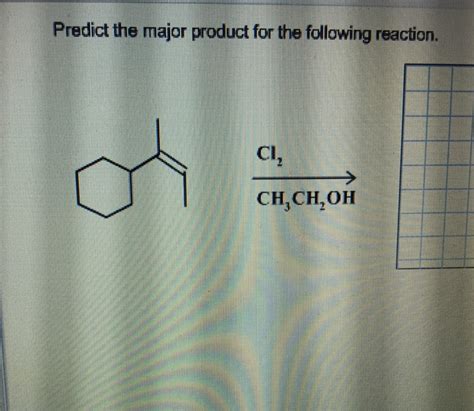
Table of Contents
Predicting the Major Product for Organic Reactions: A Comprehensive Guide
Predicting the major product of a chemical reaction is a cornerstone skill in organic chemistry. It requires a deep understanding of reaction mechanisms, functional group reactivity, and the interplay of various factors influencing reaction pathways. This comprehensive guide will delve into the strategies and principles used to accurately predict the major product for various organic reactions.
Understanding Reaction Mechanisms: The Key to Prediction
Before attempting to predict the major product, a thorough understanding of the reaction mechanism is paramount. The mechanism outlines the step-by-step process by which reactants transform into products, including the movement of electrons and the formation and breaking of bonds. This understanding allows you to identify the key intermediates and transition states, which dictate the reaction pathway and ultimately, the major product.
Common Reaction Mechanisms:
-
SN1 (Substitution Nucleophilic Unimolecular): This mechanism involves a two-step process: carbocation formation followed by nucleophilic attack. The stability of the carbocation intermediate is crucial in determining the major product. More substituted carbocations (tertiary > secondary > primary) are more stable. Therefore, SN1 reactions favor the formation of the most substituted product.
-
SN2 (Substitution Nucleophilic Bimolecular): This is a concerted, one-step mechanism where the nucleophile attacks the substrate simultaneously with the departure of the leaving group. SN2 reactions proceed with inversion of stereochemistry, meaning the configuration at the reacting carbon center is flipped. Steric hindrance plays a significant role; the reaction is favored by less hindered substrates (primary > secondary > tertiary).
-
E1 (Elimination Unimolecular): Like SN1, E1 reactions proceed via a carbocation intermediate. The reaction involves the loss of a proton and a leaving group, forming a double bond. The stability of the carbocation again dictates the major product, often leading to the most substituted alkene (Zaitsev's rule).
-
E2 (Elimination Bimolecular): This concerted mechanism involves the simultaneous removal of a proton and a leaving group by a base. The stereochemistry of the reactants is crucial; anti-periplanar geometry (180° dihedral angle) between the proton and the leaving group is preferred. Often, the most substituted alkene is the major product (Zaitsev's rule), but steric factors can influence this.
-
Addition Reactions: These reactions involve the addition of a reagent across a multiple bond (C=C or C≡C). Markovnikov's rule is frequently applied to electrophilic additions to alkenes, predicting that the electrophile adds to the carbon atom with the most hydrogen atoms. Anti-Markovnikov addition can occur in the presence of radical initiators.
Factors Influencing Product Distribution:
Several factors beyond the basic mechanism significantly impact the major product formed:
1. Substrate Structure:
The structure of the starting material is paramount. The presence of electron-donating or withdrawing groups, steric hindrance, and the nature of the leaving group all influence the reaction pathway and product distribution. For example, a bulky substrate might favor SN1 over SN2, while a good leaving group facilitates both SN1 and SN2 pathways.
2. Nucleophile/Base Strength and Steric Hindrance:
The strength and steric bulk of the nucleophile or base significantly affect the reaction outcome. A strong nucleophile favors SN2 reactions, while a bulky base may promote E2 over SN2. Weak nucleophiles often favor SN1 and E1 reactions.
3. Solvent Effects:
The solvent plays a crucial role in influencing the reaction rate and selectivity. Polar protic solvents stabilize charged intermediates, favoring SN1 and E1 reactions. Polar aprotic solvents stabilize anions, promoting SN2 reactions.
4. Temperature and Concentration:
Temperature and concentration affect the equilibrium between competing reactions. Higher temperatures often favor elimination reactions over substitution reactions. Similarly, high concentrations of reactants can favor bimolecular reactions (SN2 and E2).
5. Regioselectivity and Stereoselectivity:
Regioselectivity refers to the preferential formation of one constitutional isomer over another. Stereoselectivity refers to the preferential formation of one stereoisomer over another. Understanding these concepts is crucial in predicting the major product, particularly in reactions involving chiral centers or multiple possible isomers.
Predicting the Major Product: A Step-by-Step Approach
Predicting the major product requires a systematic approach:
-
Identify the functional groups: Determine the functional groups present in the reactants and identify the likely reaction type (e.g., substitution, elimination, addition).
-
Determine the reaction mechanism: Based on the reactants and reaction conditions, deduce the most probable mechanism (SN1, SN2, E1, E2, addition, etc.).
-
Consider substrate structure: Analyze the structure of the substrate. Identify any steric hindrance, the presence of electron-donating or withdrawing groups, and the nature of the leaving group.
-
Analyze the reagents: Evaluate the strength, steric hindrance, and nature of the nucleophile or base, and the characteristics of the solvent used.
-
Apply relevant rules: Use rules like Markovnikov's rule, Zaitsev's rule, and considerations of carbocation stability to predict the major product.
-
Consider competing reactions: Recognize that multiple reactions might compete. Determine which reaction pathway is kinetically favored or thermodynamically favored under the given conditions.
-
Draw the major product: Based on your analysis, draw the structure of the predicted major product, paying attention to regiochemistry and stereochemistry.
Examples of Predicting Major Products:
Let's illustrate this approach with examples:
Example 1: SN1 vs SN2
Consider the reaction of 2-bromo-2-methylpropane with methanol in the presence of heat. The tertiary alkyl halide favors SN1 over SN2 due to the stability of the tertiary carbocation formed. The major product will be tert-butyl methyl ether.
Example 2: E1 vs E2
The reaction of 2-chlorobutane with a strong base like potassium tert-butoxide will favor E2 elimination. The major product, according to Zaitsev's rule, would be 2-butene, the more substituted alkene. If a weaker base were used at higher temperatures, E1 elimination might become more favorable.
Example 3: Markovnikov Addition
The addition of HBr to propene follows Markovnikov's rule. The hydrogen atom adds to the carbon atom with more hydrogen atoms, resulting in 2-bromopropane as the major product.
Example 4: Anti-Markovnikov Addition
The addition of HBr to propene in the presence of a peroxide radical initiator will result in anti-Markovnikov addition, yielding 1-bromopropane as the major product. This is due to the formation of a more stable secondary radical intermediate.
Conclusion: Mastering the Art of Prediction
Predicting the major product of an organic reaction is a challenging but rewarding skill. By thoroughly understanding reaction mechanisms, analyzing the factors influencing reaction pathways, and employing a systematic approach, one can significantly improve their accuracy in predicting the major product formed. Consistent practice and a deep understanding of the underlying principles are key to mastering this important aspect of organic chemistry. Remember to always consider all potential factors and competing reactions to arrive at the most accurate prediction. This guide provides a solid foundation for developing this critical skill. Continuous learning and practice are essential to refine your predictive abilities and strengthen your understanding of organic reaction mechanisms.
Latest Posts
Latest Posts
-
Which Of The Following Describes How The Simone Framework
Mar 17, 2025
-
Download Real Communication 4th Edition Book
Mar 17, 2025
-
Which Of These Statements Accurately Describes A Dts Role
Mar 17, 2025
-
Click On The Beaker That Shows The Br Ansted Lowry Base
Mar 17, 2025
-
Label The Structures Of The Knee
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Predict The Major Product For The Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
