Click On The Beaker That Shows The Brønsted-lowry Base.
Holbox
Mar 17, 2025 · 6 min read
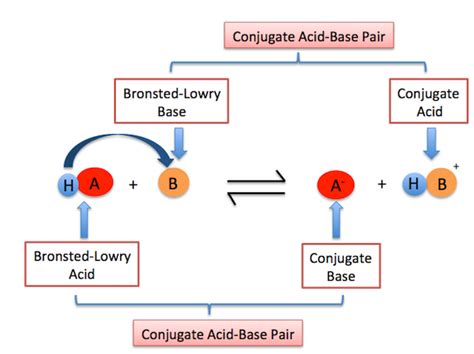
Table of Contents
Click on the Beaker that Shows the Brønsted-Lowry Base: A Deep Dive into Acid-Base Chemistry
Understanding acid-base chemistry is fundamental to many areas of science, from biochemistry and environmental science to materials science and medicine. While various definitions of acids and bases exist (Arrhenius, Lewis), the Brønsted-Lowry definition provides a particularly powerful and widely applicable framework. This article will delve into the Brønsted-Lowry definition, focusing on the identification of Brønsted-Lowry bases and offering a comprehensive exploration of their properties and reactions. We'll examine various examples, explore the concept of conjugate acid-base pairs, and discuss the implications of this theory in different chemical contexts.
What is a Brønsted-Lowry Base?
A Brønsted-Lowry base is defined as a substance that can accept a proton (H⁺) from another substance. This contrasts with the Arrhenius definition, which limits bases to hydroxide (OH⁻) ion donors. The Brønsted-Lowry definition is more inclusive, encompassing a broader range of compounds that exhibit basic properties. The key characteristic is the ability to accept a proton, leading to a change in its chemical structure and properties. This proton acceptance is crucial for the many chemical reactions in which bases participate.
Key Differences from other Acid-Base Definitions:
-
Arrhenius Base: Defined as a substance that produces hydroxide (OH⁻) ions in aqueous solution. This definition is limited as it doesn't account for bases that don't contain hydroxide ions.
-
Lewis Base: Defined as a substance that donates an electron pair. This is a broader definition than Brønsted-Lowry, including compounds that don't necessarily accept protons but can form coordinate covalent bonds by donating electrons.
Identifying a Brønsted-Lowry Base: A Practical Approach
Identifying a Brønsted-Lowry base in a given scenario requires a careful examination of the chemical species present and their potential to accept a proton. Here are some practical steps:
-
Look for a lone pair of electrons: Brønsted-Lowry bases typically possess one or more lone pairs of electrons. These lone pairs are crucial for accepting a proton, forming a new covalent bond. Molecules or ions with oxygen, nitrogen, or sulfur atoms often possess lone pairs and are likely candidates.
-
Consider the molecular structure: The molecular structure influences the availability of lone pairs and the overall basicity. Steric hindrance (bulky groups surrounding the lone pair) can reduce the accessibility of the lone pair, impacting the base's strength.
-
Analyze the reaction context: Observe the reaction equation to see which species accepts a proton. The species accepting the proton is the Brønsted-Lowry base.
Examples of Brønsted-Lowry Bases: A Diverse Group
The beauty of the Brønsted-Lowry definition lies in its ability to encompass a vast array of chemical species exhibiting basic behavior. Here are some notable examples:
1. Hydroxide Ions (OH⁻):
This is the classic example and the foundation of the Arrhenius definition. Hydroxide ions readily accept protons, forming water:
OH⁻(aq) + H⁺(aq) → H₂O(l)
2. Ammonia (NH₃):
Ammonia is a common weak base. Its lone pair on the nitrogen atom readily accepts a proton:
NH₃(aq) + H⁺(aq) → NH₄⁺(aq)
3. Amines (RNH₂, R₂NH, R₃N):
Amines are organic derivatives of ammonia, where one or more hydrogen atoms are replaced by alkyl or aryl groups (R). They are also Brønsted-Lowry bases due to the lone pair on the nitrogen atom. For example:
CH₃NH₂(aq) + H⁺(aq) → CH₃NH₃⁺(aq) (Methylamine)
4. Water (H₂O):
Water can act as both a Brønsted-Lowry acid and a Brønsted-Lowry base, showcasing its amphoteric nature. It can accept a proton to form the hydronium ion (H₃O⁺):
H₂O(l) + H⁺(aq) → H₃O⁺(aq)
5. Carbonate Ion (CO₃²⁻):
Carbonate ions possess several lone pairs on the oxygen atoms, making them capable of accepting protons. This is crucial in buffering systems and in many geochemical processes.
6. Bicarbonate Ion (HCO₃⁻):
Similar to the carbonate ion, the bicarbonate ion also acts as a Brønsted-Lowry base, capable of accepting a proton.
7. Phosphate ions (PO₄³⁻, HPO₄²⁻, H₂PO₄⁻):
Phosphate ions and their related species play vital roles in biological systems, acting as buffers and participating in numerous biochemical reactions. Their ability to accept protons makes them Brønsted-Lowry bases.
Conjugate Acid-Base Pairs: A Deeper Look
When a Brønsted-Lowry base accepts a proton, it forms its conjugate acid. The conjugate acid is the species formed after the base has accepted a proton. Conversely, when an acid donates a proton, it forms its conjugate base. These pairs are related by the difference of a single proton (H⁺).
Example: Consider the reaction of ammonia with water:
NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
- NH₃ is the base, and NH₄⁺ is its conjugate acid.
- H₂O is the acid, and OH⁻ is its conjugate base.
The strength of a base is inversely related to the strength of its conjugate acid. A strong base has a weak conjugate acid, and vice versa. This relationship is crucial for understanding acid-base equilibria.
The Strength of Brønsted-Lowry Bases: A Matter of Equilibrium
Brønsted-Lowry bases vary significantly in their strength. Strong bases readily accept protons and completely dissociate in aqueous solution. Weak bases only partially dissociate, establishing an equilibrium between the base and its conjugate acid. The extent of dissociation determines the base's strength, often quantified by its base dissociation constant (Kb). A higher Kb value indicates a stronger base.
Brønsted-Lowry Bases in Everyday Life and Beyond
Brønsted-Lowry bases are ubiquitous in everyday life and crucial in various scientific and industrial applications. Here are a few examples:
-
Baking soda (sodium bicarbonate, NaHCO₃): A common household item, it acts as a weak base, neutralizing acids and being a vital ingredient in baking.
-
Antacids: Many antacids contain Brønsted-Lowry bases to neutralize excess stomach acid, relieving heartburn and indigestion.
-
Ammonia-based cleaners: Ammonia is a common cleaning agent due to its basic properties, helping to dissolve grease and other grime.
-
Biological systems: Proteins, DNA, and RNA all contain functional groups that can act as Brønsted-Lowry bases, playing a crucial role in maintaining the proper pH and facilitating biochemical reactions.
-
Environmental chemistry: The pH of soil and water is heavily influenced by the presence of Brønsted-Lowry bases and their interactions with other chemical species.
Advanced Concepts and Applications
The Brønsted-Lowry theory has significant implications across several scientific disciplines:
-
Buffer solutions: These solutions maintain a relatively constant pH despite the addition of small amounts of acid or base. They often comprise a weak acid and its conjugate base (or a weak base and its conjugate acid), effectively resisting pH changes.
-
Titration: This analytical technique utilizes the reaction between an acid and a base to determine the concentration of an unknown solution. The Brønsted-Lowry definition is fundamental for understanding the principles of titration.
-
Catalysis: Many chemical reactions are catalyzed by Brønsted-Lowry bases, which can activate reactants and facilitate the reaction mechanism.
-
Electrochemistry: The properties of electrolytes and their interactions in electrochemical cells are explained by the Brønsted-Lowry framework.
Conclusion: The Enduring Significance of the Brønsted-Lowry Definition
The Brønsted-Lowry definition of acids and bases offers a versatile and comprehensive framework for understanding acid-base chemistry. By focusing on proton transfer, it provides a clear and consistent way to identify and characterize bases and their reactions. This understanding is crucial across numerous fields, from everyday life to cutting-edge research, emphasizing the enduring significance of this essential scientific concept. The ability to identify a Brønsted-Lowry base, therefore, is a fundamental skill for anyone involved in the study or application of chemistry.
Latest Posts
Latest Posts
-
The Highlighted Tabs Will Appear When You
Mar 17, 2025
-
Match Each Type Of Capillary To Its Most Likely Location
Mar 17, 2025
-
Rn Nutrition Online Practice 2023 A
Mar 17, 2025
-
Some Economists Argue That Early Child Care
Mar 17, 2025
-
Which Of The Following Are Included In The Opsec Cycle
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Click On The Beaker That Shows The Brønsted-lowry Base. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
