Predict The Major Organic Product Of The Reaction
Holbox
Mar 20, 2025 · 6 min read
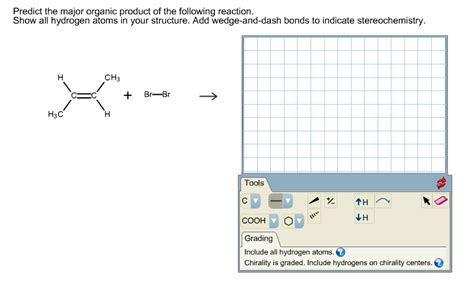
Table of Contents
Predicting the Major Organic Product of a Reaction: A Comprehensive Guide
Predicting the major organic product of a reaction is a cornerstone skill in organic chemistry. It requires a deep understanding of reaction mechanisms, functional group transformations, and the factors influencing reaction selectivity. This comprehensive guide will equip you with the tools and knowledge to confidently predict the outcome of various organic reactions. We will explore different reaction types, examine key concepts like regioselectivity and stereoselectivity, and delve into practical examples to solidify your understanding.
Understanding Reaction Mechanisms: The Foundation of Prediction
Before attempting to predict the product, you must grasp the underlying reaction mechanism. A reaction mechanism details the step-by-step process of bond breaking and bond formation, showing the movement of electrons and the formation of intermediates. Understanding the mechanism allows you to visualize the transformation of reactants into products. Different mechanisms lead to different products, even with the same starting materials and reagents.
Key Concepts in Reaction Mechanisms
-
Nucleophiles and Electrophiles: Nucleophiles are electron-rich species that donate electrons, while electrophiles are electron-deficient species that accept electrons. Understanding the interplay between nucleophiles and electrophiles is crucial for predicting the product of many reactions, such as SN1, SN2, and electrophilic aromatic substitution.
-
Carbocation Stability: Carbocations are positively charged carbon atoms. Their stability is directly related to the number of alkyl groups attached to the positively charged carbon. Tertiary carbocations are the most stable, followed by secondary, then primary, and lastly methyl carbocations. This stability influences the regioselectivity and stereoselectivity of reactions like SN1 and E1.
-
Transition States: Transition states are high-energy intermediates formed during the reaction. The energy of the transition state determines the rate of the reaction. Lower energy transition states correspond to faster reactions. Understanding transition state structures helps predict the preferred pathway and the resulting product.
-
Leaving Groups: Leaving groups are atoms or groups that depart with a pair of electrons during a reaction. Good leaving groups are weak bases and are stable as anions. Poor leaving groups hinder reactions. The nature of the leaving group significantly affects the reaction mechanism and the resulting product.
Types of Organic Reactions and Product Prediction
Organic reactions can be broadly classified into several types, each with its own set of rules and considerations for predicting the major product.
1. Nucleophilic Substitution Reactions (SN1 and SN2)
-
SN1 (Substitution Nucleophilic Unimolecular): This reaction proceeds in two steps: a carbocation intermediate is formed in the first step, followed by nucleophilic attack in the second step. The rate-determining step is the formation of the carbocation. Therefore, the stability of the carbocation dictates the regioselectivity. SN1 reactions often lead to racemization at the reaction center due to the planar nature of the carbocation.
-
SN2 (Substitution Nucleophilic Bimolecular): This reaction is a concerted process, meaning that bond breaking and bond formation occur simultaneously. The reaction rate depends on the concentrations of both the substrate and the nucleophile. SN2 reactions proceed with inversion of configuration at the stereocenter. Steric hindrance significantly affects the rate of SN2 reactions; highly hindered substrates react very slowly.
Predicting the product: For SN1, consider carbocation stability; for SN2, consider steric hindrance and inversion of configuration.
2. Elimination Reactions (E1 and E2)
-
E1 (Elimination Unimolecular): Similar to SN1, E1 reactions proceed via a carbocation intermediate. The base abstracts a proton from a carbon adjacent to the carbocation, resulting in the formation of a double bond. The stability of the carbocation governs the regioselectivity (Zaitsev's rule: more substituted alkene is favored).
-
E2 (Elimination Bimolecular): E2 reactions are concerted processes where the base abstracts a proton and the leaving group departs simultaneously. The stereochemistry of the starting material influences the stereochemistry of the product (anti-periplanar arrangement is preferred).
Predicting the product: For E1, consider carbocation stability and Zaitsev's rule; for E2, consider anti-periplanar geometry and the base strength.
3. Addition Reactions
Addition reactions involve the addition of atoms or groups to a multiple bond (double or triple bond). The type of addition reaction depends on the nature of the multiple bond and the reagents involved.
-
Electrophilic Addition: This type of addition involves the addition of an electrophile to a multiple bond. Markovnikov's rule dictates the regioselectivity in electrophilic addition to unsymmetrical alkenes. The electrophile adds to the carbon atom bearing the greater number of hydrogen atoms.
-
Nucleophilic Addition: This type of addition involves the addition of a nucleophile to a multiple bond. Nucleophilic addition to carbonyl compounds is a common example, leading to the formation of alcohols or other functional groups.
Predicting the product: For electrophilic addition, apply Markovnikov's rule; for nucleophilic addition, consider the reactivity of the nucleophile and the electrophile.
4. Oxidation and Reduction Reactions
Oxidation involves the loss of electrons, while reduction involves the gain of electrons. Many oxidizing and reducing agents are available, each with its own selectivity and reactivity.
-
Oxidation of Alcohols: Primary alcohols can be oxidized to aldehydes or carboxylic acids, while secondary alcohols are oxidized to ketones. Tertiary alcohols are resistant to oxidation.
-
Reduction of Carbonyl Compounds: Carbonyl compounds (aldehydes and ketones) can be reduced to alcohols using reducing agents like lithium aluminum hydride (LiAlH4) or sodium borohydride (NaBH4).
Predicting the product: Consider the type of alcohol or carbonyl compound and the strength of the oxidizing or reducing agent.
5. Electrophilic Aromatic Substitution
Electrophilic aromatic substitution involves the substitution of a hydrogen atom on an aromatic ring with an electrophile. The directing effects of substituents on the aromatic ring influence the regioselectivity of the reaction. Activating groups (e.g., -OH, -NH2) are ortho/para directing, while deactivating groups (e.g., -NO2, -COOH) are meta directing.
Predicting the product: Consider the directing effect of substituents on the aromatic ring and the reactivity of the electrophile.
Factors Influencing Reaction Selectivity
Reaction selectivity refers to the preference of a reaction to form one product over another. Several factors influence selectivity:
-
Steric effects: Bulky groups can hinder the approach of reagents, influencing the reaction pathway and the resulting product.
-
Electronic effects: Electron-donating or electron-withdrawing groups can affect the reactivity of the substrate and the selectivity of the reaction.
-
Temperature: Temperature can affect the reaction rate and the relative rates of competing reactions.
-
Solvent: The solvent can influence the reaction rate and selectivity by stabilizing or destabilizing intermediates.
Practical Examples and Problem Solving
Let's consider some practical examples to illustrate how to predict the major organic product:
Example 1: SN2 Reaction
Reactant: Bromomethane (CH3Br) Reagent: Sodium hydroxide (NaOH) in ethanol
Prediction: The hydroxide ion (OH-) acts as a nucleophile and attacks the carbon atom bearing the bromine atom. This is an SN2 reaction, resulting in inversion of configuration. The major product is methanol (CH3OH).
Example 2: E1 Reaction
Reactant: 2-bromo-2-methylpropane Reagent: Ethanol at elevated temperature
Prediction: The reaction proceeds via a carbocation intermediate. The most stable carbocation is formed, followed by elimination of a proton to give 2-methylpropene (isobutylene) as the major product (Zaitsev's rule).
Example 3: Electrophilic Aromatic Substitution
Reactant: Benzene Reagent: Nitric acid (HNO3) and sulfuric acid (H2SO4)
Prediction: The nitronium ion (NO2+) acts as the electrophile and attacks the benzene ring. The product is nitrobenzene.
Conclusion
Predicting the major organic product of a reaction is a crucial skill in organic chemistry. It requires a thorough understanding of reaction mechanisms, functional group transformations, and the factors influencing reaction selectivity. By mastering these concepts and practicing with various examples, you can significantly enhance your ability to accurately predict the outcome of organic reactions. Remember that practice is key to mastering this skill; work through numerous problems, and consult reference materials when needed. Consistent effort will lead to increased confidence and success in predicting the products of organic reactions.
Latest Posts
Latest Posts
-
Drag The Appropriate Labels To Their Respective Targets Facial Nerve
Mar 21, 2025
-
How Are An Interval And A Segment Different
Mar 21, 2025
-
The Two Main Types Of Data Are
Mar 21, 2025
-
What Is Meant By The Phrase Spreading The Overhead
Mar 21, 2025
-
The Hydrolysis Of Esters Amides And Nitriles
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Predict The Major Organic Product Of The Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
