Match Each Titration Term With Its Definition
Holbox
Mar 16, 2025 · 6 min read
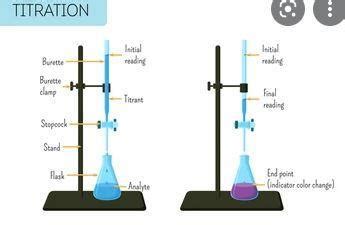
Table of Contents
Matching Titration Terms with Their Definitions: A Comprehensive Guide
Titration, a cornerstone technique in analytical chemistry, allows precise determination of a substance's concentration. Mastering titration requires a solid understanding of its terminology. This comprehensive guide meticulously defines key titration terms, enhancing your understanding and proficiency in this essential laboratory procedure. We will cover everything from the basics to more advanced concepts, ensuring a thorough understanding of the language of titration.
Fundamental Titration Terms
Let's begin with the fundamental concepts that form the bedrock of titration:
1. Titration:
The process of gradually adding a solution of known concentration (the titrant) to a solution of unknown concentration (the analyte) until the chemical reaction between them is complete. This point of completion is called the equivalence point.
2. Titrant (Standard Solution):
A solution of precisely known concentration used to react with the analyte in a titration. The concentration of the titrant is usually determined through a process called standardization, which we'll discuss later. Accuracy in preparing and knowing the concentration of the titrant is paramount for accurate results in a titration.
3. Analyte:
The substance whose concentration is being determined in a titration. The analyte can be a variety of substances, from acids and bases to various ions and compounds. Proper preparation and handling of the analyte are crucial for obtaining reliable data.
4. Equivalence Point:
The point in a titration where the amount of titrant added is stoichiometrically equivalent to the amount of analyte present. This means that the moles of titrant react completely with the moles of analyte according to the balanced chemical equation. It's an important theoretical point, often not directly observable.
5. End Point:
The point in a titration where a noticeable change occurs, signaling the completion of the reaction. This change is usually indicated by a color change from an indicator or a change in pH measured by a pH meter. The end point is an experimental observation, and ideally, it should be as close as possible to the equivalence point.
Indicators and Their Role in Titration
Indicators are crucial in many titrations. Let's explore these terms:
6. Indicator:
A substance that changes color at or near the equivalence point of a titration. Indicators are typically weak acids or bases that exhibit different colors in their acidic and basic forms. The choice of indicator depends heavily on the specific titration being performed, as different indicators change color at different pH ranges.
7. pH:
A measure of the hydrogen ion concentration in a solution. It indicates the acidity or basicity of the solution on a scale of 0 to 14, where 7 is neutral, values below 7 are acidic, and values above 7 are basic (alkaline). pH is a crucial factor in determining the equivalence point in acid-base titrations.
8. pH Change at Equivalence Point:
The magnitude of the pH change around the equivalence point. A sharp pH change indicates a well-defined equivalence point, making it easier to accurately determine the end point. The sharpness of this change varies depending on the strength of the acid and base involved in the titration.
Titration Techniques and Related Terms
Different titration techniques exist, each with its specific terminology:
9. Acid-Base Titration:
A titration involving a reaction between an acid and a base. This is a very common type of titration used to determine the concentration of acidic or basic solutions. The equivalence point is often determined by observing a sharp change in pH.
10. Redox Titration:
A titration involving an oxidation-reduction reaction. In this type of titration, the titrant is an oxidizing or reducing agent, and the equivalence point is detected by a change in the oxidation state of the analyte or a change in potential measured with a suitable electrode.
11. Complexometric Titration:
A titration where the reaction involves the formation of a complex ion. A common example is the titration of metal ions using EDTA (ethylenediaminetetraacetic acid), which forms stable complexes with many metal ions. These titrations are often used to determine the concentration of metal ions in solution.
12. Precipitation Titration:
A titration involving a precipitation reaction, where a solid product is formed. The equivalence point is often determined by observing the appearance or disappearance of a precipitate. Examples include titrations using silver nitrate to determine the concentration of halide ions.
13. Standardisation (Standardization):
The process of determining the exact concentration of a titrant solution. This is typically done by titrating the titrant against a primary standard, a substance of known high purity and accurately known molar mass. Standardization ensures the accuracy of the titration results.
14. Primary Standard:
A highly pure substance with a precisely known chemical composition used to standardize a titrant. Primary standards must be stable, non-hygroscopic (not easily absorbing moisture), and have a high molar mass to minimize weighing errors.
Advanced Titration Concepts
Let's delve into some more advanced concepts:
15. Titration Curve:
A graph showing the change in pH (or other relevant property) as a function of the volume of titrant added. Titration curves are valuable tools for visualizing the progress of a titration, identifying the equivalence point, and selecting an appropriate indicator.
16. Half-Equivalence Point:
The point in a titration where half of the analyte has reacted with the titrant. In acid-base titrations, the pH at the half-equivalence point is equal to the pKa (negative logarithm of the acid dissociation constant) of the weak acid being titrated. This is useful for determining the pKa of a weak acid.
17. Back Titration:
A titration technique where an excess of titrant is added to the analyte, and the remaining unreacted titrant is then titrated with a second standard solution. This technique is particularly useful when the reaction between the analyte and titrant is slow or incomplete.
18. Blank Titration:
A titration performed without the analyte present. This helps to correct for any impurities or interfering substances in the titrant or other reagents used in the titration. The volume of titrant used in the blank titration is subtracted from the volume used in the actual titration.
Practical Considerations and Troubleshooting
19. Systematic Error:
Errors in titration due to factors like inaccurate equipment calibration, impure reagents, or incorrect procedures. Minimizing systematic errors is crucial for obtaining accurate results.
20. Random Error:
Errors due to unpredictable variations in the experimental setup, such as slight variations in reading the burette. Random errors can be minimized by careful technique and performing multiple titrations.
21. Burette:
A graduated glass tube with a stopcock at the bottom used to precisely deliver variable volumes of titrant. Proper use and reading of the burette are critical for accurate titration.
22. Pipette:
A glass tube used to accurately transfer a specific volume of liquid. Using pipettes ensures consistent volumes of analyte in each titration.
23. Conical Flask (Erlenmeyer Flask):
A type of flask with a conical body and a narrow neck used to contain the analyte during titration. The narrow neck prevents splashing during the titration process.
Conclusion: Mastering the Language of Titration
This comprehensive guide has explored a wide range of terms crucial to understanding and performing titrations. From fundamental definitions to advanced concepts and practical considerations, a firm grasp of this terminology is essential for success in analytical chemistry. By understanding the nuances of each term, you can confidently navigate the complexities of titration and accurately determine the concentration of unknown substances. Remember to always practice safe laboratory procedures and ensure proper calibration and maintenance of equipment for reliable results. Through careful attention to detail and a solid understanding of the principles involved, you can unlock the power of titration and its applications in diverse scientific fields.
Latest Posts
Latest Posts
-
What Is Hootsuite Inbox Is Used For Pick Three
Mar 16, 2025
-
Which Distribution Channel Drives Results Fastest
Mar 16, 2025
-
A Constraint In A Decision Is A Restriction Placed On
Mar 16, 2025
-
A Circuit Is Constructed With Six Resistors And Two Batteries
Mar 16, 2025
-
The Decomposition Of N2o5 Can Be Described By The Equation
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about Match Each Titration Term With Its Definition . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
