Lithium Diisopropylamide Is A Strong Nonnucleophilic Base
Holbox
Mar 29, 2025 · 5 min read
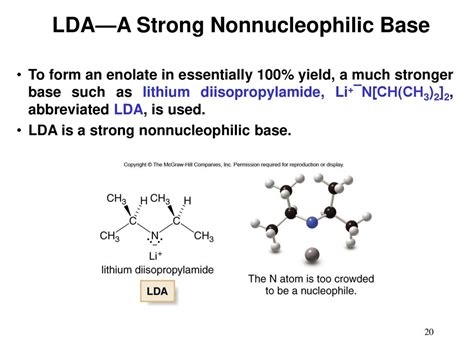
Table of Contents
- Lithium Diisopropylamide Is A Strong Nonnucleophilic Base
- Table of Contents
- Lithium Diisopropylamide (LDA): A Powerful Non-Nucleophilic Base in Organic Synthesis
- Understanding the Non-Nucleophilic Nature of LDA
- Comparing LDA to Other Strong Bases
- Preparation of Lithium Diisopropylamide (LDA)
- The Reaction Mechanism:
- Applications of LDA in Organic Synthesis
- 1. Enolate Formation:
- 2. Directed Aldol Reactions:
- 3. Preparation of Organolithium Reagents:
- 4. α-Halogenation of Ketones:
- 5. Preparation of Functionalized Heterocycles:
- Practical Considerations for Using LDA
- 1. Anhydrous Conditions:
- 2. Low Temperatures:
- 3. Stoichiometry:
- 4. Solvent Selection:
- 5. Addition Rate:
- 6. Work-up Procedures:
- Safety Precautions:
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Lithium Diisopropylamide (LDA): A Powerful Non-Nucleophilic Base in Organic Synthesis
Lithium diisopropylamide (LDA), with its chemical formula [(CH₃)₂CH]₂NLi, stands as a cornerstone reagent in organic chemistry, renowned for its exceptional utility as a strong non-nucleophilic base. This characteristic allows it to deprotonate a wide array of weakly acidic compounds without simultaneously participating in nucleophilic substitution reactions, a crucial distinction that opens up diverse synthetic possibilities. Understanding LDA's unique properties, preparation, and applications is fundamental for any organic chemist. This comprehensive guide delves deep into the intricacies of LDA, exploring its mechanism of action, practical considerations for its use, and its pivotal role in various synthetic transformations.
Understanding the Non-Nucleophilic Nature of LDA
The power of LDA lies in its ability to act as a strong base while simultaneously minimizing its nucleophilicity. This seemingly paradoxical property stems from its steric bulk. The isopropyl groups flanking the nitrogen atom create significant steric hindrance around the lithium-nitrogen bond. This bulkiness effectively shields the nitrogen atom, greatly reducing its ability to participate in nucleophilic attacks. Consequently, LDA preferentially acts as a base, abstracting protons from acidic substrates rather than engaging in nucleophilic addition or substitution reactions.
Comparing LDA to Other Strong Bases
Other strong bases, such as sodium hydride (NaH) or potassium tert-butoxide (t-BuOK), also exhibit high basicity but are significantly more nucleophilic. Their smaller size and less sterically hindered nature allow them to readily participate in nucleophilic reactions, limiting their applicability in certain synthetic transformations where selectivity is crucial. LDA's unique steric profile addresses this limitation, offering a powerful tool for selective deprotonation.
Preparation of Lithium Diisopropylamide (LDA)
LDA is typically prepared in situ (within the reaction vessel) immediately prior to its use. This is because LDA is moisture- and air-sensitive, decomposing readily upon exposure. Common preparation methods involve the reaction of diisopropylamine with a strong alkyllithium reagent, such as n-butyllithium (n-BuLi).
The Reaction Mechanism:
The reaction proceeds via a simple acid-base reaction:
(CH₃)₂CHNH₂ + n-BuLi → [(CH₃)₂CH]₂NLi + n-BuH
n-Butane (n-BuH) is a byproduct that is readily removed. The reaction is typically carried out under an inert atmosphere (e.g., nitrogen or argon) at low temperatures, often between -78°C and 0°C, to maximize yield and control the reaction. Temperature control is critical in preventing undesired side reactions.
Applications of LDA in Organic Synthesis
LDA's versatility has made it an indispensable reagent in numerous synthetic transformations. Its ability to selectively deprotonate weakly acidic compounds without competing nucleophilic reactions renders it particularly useful in reactions where chemoselectivity is paramount.
1. Enolate Formation:
One of LDA's most prominent uses is in the formation of enolates from ketones, aldehydes, and esters. LDA's strong basicity and non-nucleophilic character allow for the selective deprotonation of the α-carbon, generating a kinetic enolate. This is crucial for controlling regioselectivity, particularly in unsymmetrical carbonyl compounds. The kinetic enolate, being less thermodynamically stable, is typically formed faster at lower temperatures.
Kinetic vs. Thermodynamic Enolates:
The choice between kinetic and thermodynamic enolates depends on the desired product. Kinetic enolates are formed faster at lower temperatures and often react faster with electrophiles. Thermodynamic enolates, more stable and formed at higher temperatures, may be preferred in specific cases. LDA favors the formation of kinetic enolates.
2. Directed Aldol Reactions:
LDA plays a critical role in directed aldol reactions. By selectively deprotonating a specific carbonyl compound, LDA allows for the controlled addition of the resulting enolate to another aldehyde or ketone, yielding β-hydroxy carbonyl compounds. This controlled addition is essential in achieving high stereoselectivity and yields.
3. Preparation of Organolithium Reagents:
LDA can also serve as a base in the preparation of various organolithium reagents. By deprotonating suitable substrates, it facilitates the formation of reactive organolithium species which can subsequently participate in further synthetic steps.
4. α-Halogenation of Ketones:
LDA is used to generate enolates of ketones, which can then react with halogenating agents to yield α-halogenated ketones. This is a critical reaction in synthesizing a variety of valuable compounds.
5. Preparation of Functionalized Heterocycles:
LDA enables the formation of various functionalized heterocycles through the generation and subsequent intramolecular cyclization of appropriately functionalized enolates. The ability of LDA to achieve selective deprotonation allows for the formation of highly specific heterocyclic structures.
Practical Considerations for Using LDA
Successful utilization of LDA requires careful consideration of several crucial factors.
1. Anhydrous Conditions:
LDA is extremely sensitive to moisture and air. All glassware and solvents must be meticulously dried before use. The reaction must be carried out under an inert atmosphere (e.g., nitrogen or argon).
2. Low Temperatures:
Reactions using LDA are typically performed at low temperatures, usually between -78°C and 0°C. This helps control the reaction rate, prevents side reactions, and favors the formation of kinetic enolates.
3. Stoichiometry:
The stoichiometry of the reaction is important. Typically, one equivalent of LDA is used for each equivalent of substrate to be deprotonated. However, in some cases, an excess of LDA may be needed to ensure complete conversion.
4. Solvent Selection:
Appropriate solvent selection is crucial for LDA reactions. Tetrahydrofuran (THF) is a common solvent, but other aprotic solvents may be used depending on the specific reaction.
5. Addition Rate:
The rate of addition of LDA and the substrate must be carefully controlled to prevent side reactions and maximize yield. Slow addition is generally preferred.
6. Work-up Procedures:
The work-up procedure must be carefully chosen to avoid decomposition of the product. Careful quenching of the reaction mixture with aqueous acid or base is necessary.
Safety Precautions:
LDA is a strong base and must be handled with caution. Appropriate personal protective equipment (PPE), such as gloves, goggles, and lab coats, should be worn at all times. The reaction should be carried out in a well-ventilated area or fume hood.
Conclusion
Lithium diisopropylamide (LDA) stands as a powerful and versatile reagent in organic synthesis, distinguished by its exceptional strength as a non-nucleophilic base. Its ability to selectively deprotonate weakly acidic compounds, while avoiding nucleophilic side reactions, is critical in various synthetic transformations, including enolate formation, directed aldol reactions, and the synthesis of heterocycles. Understanding LDA's properties, preparation, and application necessitates careful attention to anhydrous conditions, temperature control, and appropriate work-up procedures. Its utility in achieving selective and controlled reactivity makes it an indispensable tool in the modern organic chemist's arsenal. The continued exploration of LDA's reactivity and applications promises further advancements in organic synthesis.
Latest Posts
Latest Posts
-
The Bargaining Leverage Of Suppliers Is Stronger When
Apr 02, 2025
-
Experiment 10 Analysis Of An Aluminum Zinc Alloy
Apr 02, 2025
-
Correctly Label The Following Anatomical Parts Of A Kidney
Apr 02, 2025
-
Identify The Possible Double Jeopardy For Elderly Ethnic Minority Individuals
Apr 02, 2025
-
How Many Moles Are In 15 Grams Of Lithium
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Lithium Diisopropylamide Is A Strong Nonnucleophilic Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
