Lewis Dot Structure For Aso4 3
Holbox
Mar 22, 2025 · 5 min read
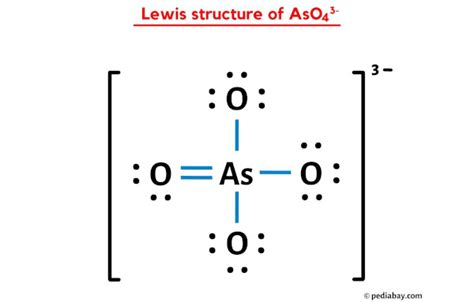
Table of Contents
- Lewis Dot Structure For Aso4 3
- Table of Contents
- Lewis Dot Structure for AsO₄³⁻: A Comprehensive Guide
- Understanding Lewis Dot Structures
- Step-by-Step Construction of the AsO₄³⁻ Lewis Structure
- Step 1: Counting Valence Electrons
- Step 2: Identifying the Central Atom
- Step 3: Connecting Atoms with Single Bonds
- Step 4: Completing Octet for Outer Atoms
- Step 5: Checking for Octet Rule on Central Atom
- Step 6: Formal Charge Calculation
- Resonance Structures of AsO₄³⁻
- Geometry and Hybridization of AsO₄³⁻
- Importance and Applications of AsO₄³⁻
- Comparing AsO₄³⁻ to PO₄³⁻
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Lewis Dot Structure for AsO₄³⁻: A Comprehensive Guide
The arsenate ion, AsO₄³⁻, is a crucial component in various biochemical processes and environmental systems. Understanding its structure is fundamental to comprehending its reactivity and role in these systems. This detailed guide will explore the Lewis dot structure of AsO₄³⁻, delving into the step-by-step construction, formal charges, resonance structures, and the overall geometry and bonding characteristics. We'll also touch upon its applications and importance.
Understanding Lewis Dot Structures
Before diving into the AsO₄³⁻ structure, let's refresh our understanding of Lewis dot structures. These diagrams visually represent the valence electrons of atoms and their arrangement in molecules or ions. They are crucial for predicting molecular geometry, bonding types (covalent, ionic), and overall properties. Key elements of a Lewis structure include:
- Valence Electrons: The outermost electrons of an atom, which participate in bonding.
- Octet Rule: Atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight valence electrons (except for hydrogen and helium, which aim for two).
- Lone Pairs: Pairs of valence electrons not involved in bonding.
- Bonding Pairs: Pairs of valence electrons shared between atoms, forming covalent bonds.
Step-by-Step Construction of the AsO₄³⁻ Lewis Structure
Let's build the Lewis structure for the arsenate ion (AsO₄³⁻) step-by-step:
Step 1: Counting Valence Electrons
- Arsenic (As) has 5 valence electrons.
- Oxygen (O) has 6 valence electrons each, and there are four oxygen atoms.
- The 3- charge indicates the presence of three extra electrons.
Total valence electrons = 5 + (4 × 6) + 3 = 32 electrons
Step 2: Identifying the Central Atom
Arsenic (As) is the least electronegative atom and will act as the central atom.
Step 3: Connecting Atoms with Single Bonds
Connect the central arsenic atom to each of the four oxygen atoms with single bonds. This uses 8 electrons (4 bonds × 2 electrons/bond).
Step 4: Completing Octet for Outer Atoms
Distribute the remaining electrons (32 - 8 = 24 electrons) to the oxygen atoms to satisfy the octet rule. Each oxygen atom needs 6 more electrons to achieve an octet (already has 2 from the single bond). This step uses all 24 remaining electrons.
Step 5: Checking for Octet Rule on Central Atom
The arsenic atom currently only has 8 electrons (4 single bonds × 2 electrons/bond). The octet rule is satisfied for both the central and outer atoms in this structure.
Step 6: Formal Charge Calculation
It's crucial to calculate the formal charge for each atom to ensure the most stable Lewis structure. The formal charge is calculated using the formula:
Formal charge = (Valence electrons) - (Non-bonding electrons) - (1/2 × Bonding electrons)
- Arsenic (As): Formal charge = 5 - 0 - (1/2 × 8) = +1
- Oxygen (O): Formal charge = 6 - 6 - (1/2 × 2) = -1 (for each oxygen atom)
The overall charge of the ion (+1 + 4(-1)) = -3, which matches the ion's charge. However, a more stable structure can be obtained.
Resonance Structures of AsO₄³⁻
The Lewis structure we've constructed is just one possible representation of the arsenate ion. Due to the possibility of double bonding between arsenic and oxygen atoms, the AsO₄³⁻ ion exhibits resonance. Resonance structures represent different ways the electrons can be distributed while still maintaining the overall connectivity of the atoms. In the case of AsO₄³⁻, we can draw multiple resonance structures with double bonds between arsenic and one or more oxygen atoms. The actual structure of the ion is a hybrid of these resonance structures, meaning the electrons are delocalized across multiple bonds.
Illustrating all possible resonance structures is lengthy, but here’s the idea: each resonance structure would have one or more double bonds between As and O, and the negative charge will be distributed between the oxygen atoms involved in single bonds. Each structure contributes to the overall resonance hybrid.
Geometry and Hybridization of AsO₄³⁻
The arsenate ion exhibits a tetrahedral geometry. The central arsenic atom is surrounded by four oxygen atoms arranged in a tetrahedron. This geometry is consistent with the sp³ hybridization of the arsenic atom. The four sp³ hybrid orbitals of arsenic overlap with the p orbitals of the four oxygen atoms, forming four sigma bonds.
Importance and Applications of AsO₄³⁻
Arsenate (AsO₄³⁻) plays a significant role in various contexts:
- Biochemistry: Arsenate is a structural analog of phosphate (PO₄³⁻). This similarity allows it to interfere with phosphate-dependent biochemical processes. It can substitute for phosphate in several metabolic pathways, disrupting cellular functions.
- Environmental Science: Arsenate is a prevalent contaminant in groundwater, posing serious health risks. Understanding its behavior and reactivity in environmental systems is crucial for remediation efforts.
- Industrial Applications: Arsenate is used in some industrial processes, though its use is decreasing due to its toxicity.
Comparing AsO₄³⁻ to PO₄³⁻
Both arsenate (AsO₄³⁻) and phosphate (PO₄³⁻) have similar tetrahedral structures. However, arsenic's larger size and weaker bonding compared to phosphorus lead to different properties and reactivities. Arsenate's ability to substitute for phosphate highlights its biological impact, often causing disruption in metabolic processes.
Conclusion
The Lewis dot structure of AsO₄³⁻ is a fundamental tool for understanding the bonding, geometry, and properties of this important ion. While a single Lewis structure can be drawn, the resonance structures and the delocalization of electrons paint a more complete picture. This understanding is critical for appreciating the arsenate ion's role in various biological, environmental, and industrial contexts. Remember that constructing and analyzing Lewis structures is a crucial skill for anyone studying chemistry, particularly in the realms of inorganic chemistry and biochemistry. The ability to visualize electron distribution facilitates predictions about molecular properties and reactivity.
Latest Posts
Latest Posts
-
Which Document Name Indicates That It Does Not Contain Macros
Mar 24, 2025
-
Provide The Correct Systematic Name For The Compound Shown Here
Mar 24, 2025
-
When Does Title To Stolen Goods Transfer
Mar 24, 2025
-
Draw The Product Of The E2 Reaction Shown Below
Mar 24, 2025
-
What Is The Normal Boiling Point For Iodine
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Lewis Dot Structure For Aso4 3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
