Provide The Correct Systematic Name For The Compound Shown Here
Holbox
Mar 24, 2025 · 7 min read
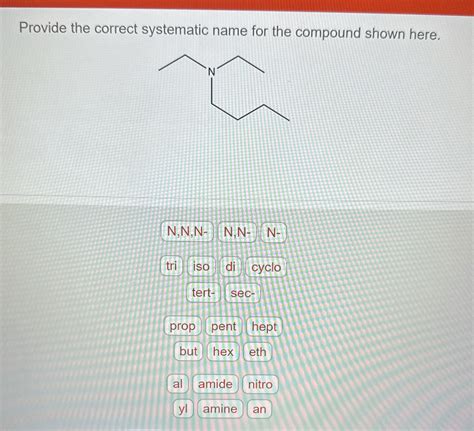
Table of Contents
- Provide The Correct Systematic Name For The Compound Shown Here
- Table of Contents
- Providing the Correct Systematic Name for a Chemical Compound: A Comprehensive Guide
- Understanding the Fundamentals of IUPAC Nomenclature
- 1. Identifying the Parent Chain or Ring:
- 2. Identifying Functional Groups:
- 3. Numbering the Carbon Chain or Ring:
- 4. Naming Substituents:
- 5. Combining the Information:
- Naming Alkanes, Alkenes, and Alkynes
- Naming Compounds with Functional Groups
- Handling Multiple Functional Groups and Complex Structures
- Illustrative Examples and Step-by-Step Naming
- Importance of Accurate Nomenclature
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Providing the Correct Systematic Name for a Chemical Compound: A Comprehensive Guide
Determining the correct systematic name for a chemical compound is crucial in chemistry. Systematic nomenclature, unlike common names, provides a unique and unambiguous identifier for each molecule, regardless of its source or trivial name. This ensures clarity and prevents confusion, particularly when dealing with complex molecules or in international collaborations. This article will explore the principles of systematic nomenclature, focusing on the IUPAC (International Union of Pure and Applied Chemistry) system, widely regarded as the standard. We'll delve into the step-by-step process of naming various types of compounds, including alkanes, alkenes, alkynes, alcohols, aldehydes, ketones, carboxylic acids, and more complex structures containing multiple functional groups.
Understanding the Fundamentals of IUPAC Nomenclature
The IUPAC system relies on a set of well-defined rules to create unique names for chemical compounds. These rules consider the parent chain or ring, the functional groups present, and the position of substituents. Understanding these elements is fundamental to correctly naming any compound.
1. Identifying the Parent Chain or Ring:
The parent chain or ring is the longest continuous carbon chain or the most stable ring system within the molecule. This chain or ring forms the basis of the compound's name. For example, in a branched alkane, you must first identify the longest continuous carbon chain, even if it requires changing the orientation of the molecule on paper.
2. Identifying Functional Groups:
Functional groups are specific atoms or groups of atoms within a molecule that confer characteristic chemical properties. Common functional groups include:
- Hydroxyl (-OH): Alcohols
- Carbonyl (C=O): Aldehydes, ketones, carboxylic acids, esters, amides
- Carboxyl (-COOH): Carboxylic acids
- Amino (-NH2): Amines
- Ether (-O-): Ethers
- Halo (F, Cl, Br, I): Haloalkanes
The presence and location of functional groups significantly influence the compound's name and properties.
3. Numbering the Carbon Chain or Ring:
Once the parent chain or ring is identified, the carbons are numbered to assign locations to substituents and functional groups. Numbering typically starts from the end closest to the highest-priority functional group (e.g., the carboxyl group in carboxylic acids). The goal is to achieve the lowest possible set of locants (numbers).
4. Naming Substituents:
Substituents are atoms or groups attached to the parent chain or ring. These are named alphabetically and their positions are indicated by numbers. Prefixes like di, tri, tetra are used to indicate multiple occurrences of the same substituent.
5. Combining the Information:
Finally, the information gathered — parent chain/ring, functional groups, substituents, and their locations — is combined according to specific rules to generate the systematic name.
Naming Alkanes, Alkenes, and Alkynes
Alkanes, alkenes, and alkynes are hydrocarbons containing only single, double, and triple bonds, respectively. Their systematic names are based on the number of carbon atoms in the parent chain.
Alkanes: The parent alkane names are methane (1 carbon), ethane (2 carbons), propane (3 carbons), butane (4 carbons), pentane (5 carbons), hexane (6 carbons), heptane (7 carbons), octane (8 carbons), nonane (9 carbons), and decane (10 carbons). Branched alkanes are named by identifying the longest continuous carbon chain, then naming the substituents (alkyl groups) attached to it.
Example: 2-methylbutane (a methyl group on the second carbon of a butane chain)
Alkenes: Alkenes are named similarly to alkanes, but with the suffix "-ene" and a number indicating the position of the double bond. The numbering starts from the end closest to the double bond.
Example: 2-pentene (a double bond between carbons 2 and 3 in a pentane chain)
Alkynes: Alkynes follow the same pattern, using the suffix "-yne" to denote the triple bond.
Example: 1-butyne (a triple bond between carbons 1 and 2 in a butane chain)
Naming Compounds with Functional Groups
Compounds containing functional groups are named by identifying the parent chain or ring containing the functional group and then adding the appropriate suffix or prefix based on the functional group's priority.
Alcohols: The suffix "-ol" is used for alcohols, with a number indicating the position of the hydroxyl group.
Example: 2-propanol (a hydroxyl group on the second carbon of a propane chain)
Aldehydes: The suffix "-al" is used for aldehydes. The aldehyde group is always at the end of the chain, so no number is needed.
Example: Butanal (an aldehyde with four carbon atoms)
Ketones: The suffix "-one" is used for ketones, with a number indicating the position of the carbonyl group.
Example: 3-pentanone (a carbonyl group on the third carbon of a pentane chain)
Carboxylic Acids: The suffix "-oic acid" is used for carboxylic acids. The carboxyl group is always at the end of the chain, so no number is needed.
Example: Butanoic acid (a carboxylic acid with four carbon atoms)
Esters: Esters are named by identifying the alkyl group attached to the oxygen and the parent carboxylic acid.
Example: Ethyl acetate (ester derived from acetic acid and ethanol)
Amines: Amines are named by identifying the alkyl groups attached to the nitrogen atom, using prefixes like N- to indicate substitution on the nitrogen atom.
Example: N,N-Dimethylethanamine (two methyl groups attached to the nitrogen of ethanamine)
Handling Multiple Functional Groups and Complex Structures
When dealing with molecules containing multiple functional groups, the priority order of functional groups determines which one gets the suffix and which ones become prefixes. The IUPAC system provides a detailed priority list. For instance, carboxylic acids have higher priority than alcohols or ketones. Complex structures often require a systematic approach, breaking them down into simpler parts and assembling the name step-by-step. Ring systems have their own set of rules for naming, often involving prefixes to indicate the ring type and suffixes to represent functional groups attached to it.
Illustrative Examples and Step-by-Step Naming
Let's illustrate the naming process with a few more complex examples. This will demonstrate how to systematically apply the rules we have discussed.
Example 1: Consider a molecule with a longest carbon chain of six carbons containing a double bond on carbon 3, a methyl group on carbon 2, and a hydroxyl group on carbon 4.
- Identify the parent chain: Hexene (6 carbons, double bond)
- Locate the double bond: The double bond is between carbons 3 and 4.
- Number the chain: Number the chain from the end closest to the double bond (to get the lowest locants).
- Identify and locate substituents: Methyl group on carbon 2 and a hydroxyl group on carbon 4.
- Combine information: The name becomes 4-hydroxy-2-methylhex-3-ene. Note that "hydroxy" gets a lower priority than the alkene (hence it is a prefix) and numbers are arranged with the lowest possible value for each functional group.
Example 2: Consider a molecule with a benzene ring containing a methyl group at position 1, a nitro group at position 3, and a carboxylic acid at position 5.
- Identify the parent structure: Benzene
- Number the ring: We number to minimize the locants.
- Identify and locate substituents: Methyl (1), nitro (3), carboxylic acid (5).
- Combine information: The carboxylic acid has the highest priority, so the suffix will be "-oic acid." The other substituents are named as prefixes. The name will be 3-nitro-1-methylbenzoic acid.
Importance of Accurate Nomenclature
The accurate naming of chemical compounds is essential for various reasons:
- Unambiguous Communication: Systematic names avoid ambiguity, ensuring that chemists worldwide understand the same molecule when discussing it.
- Database Searches: Chemical databases and literature searches rely on accurate nomenclature to retrieve relevant information.
- Intellectual Property: Correct naming is crucial for patenting new compounds and protecting intellectual property rights.
- Safety and Regulation: Accurate nomenclature is vital in safety data sheets and regulatory documents for proper handling and use of chemicals.
Conclusion
Mastering IUPAC nomenclature is a cornerstone of chemical education and practice. While the rules may seem intricate at first, a systematic approach—identifying the parent chain, locating functional groups, numbering the carbon atoms, and applying the correct prefixes and suffixes—will allow you to confidently name a wide range of organic and inorganic compounds. Consistent practice and familiarity with the priority rules will ultimately enhance your understanding of chemical structures and facilitate clear and effective communication within the scientific community. Remember to always prioritize clarity, accuracy, and adherence to IUPAC guidelines for unambiguous representation of chemical compounds.
Latest Posts
Latest Posts
-
If The Minimum Wage Is A Binding Price Floor
Mar 27, 2025
-
One Principle Of Civil Engineering Is That When You Design
Mar 27, 2025
-
Jack Is Considering Selling His Elliptical Machine
Mar 27, 2025
-
Label The Diagram Of A Growing Polynucleotide Chain
Mar 27, 2025
-
Devise A 4 Step Synthesis Of 1 Bromopropane From 2 Bromopropane
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Provide The Correct Systematic Name For The Compound Shown Here . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
