Devise A 4-step Synthesis Of 1-bromopropane From 2-bromopropane
Holbox
Mar 27, 2025 · 4 min read
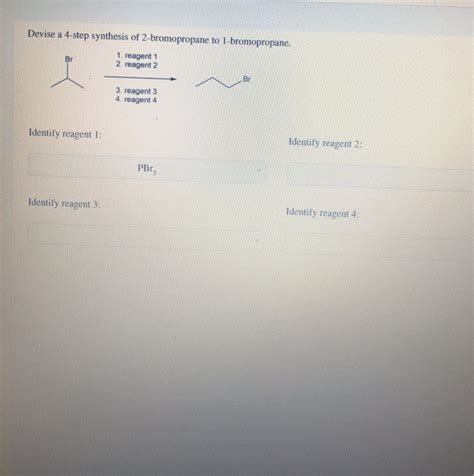
Table of Contents
- Devise A 4-step Synthesis Of 1-bromopropane From 2-bromopropane
- Table of Contents
- Devising a 4-Step Synthesis of 1-Bromopropane from 2-Bromopropane
- Understanding the Challenge: Steric Hindrance and Regioselectivity
- The Four-Step Synthesis: A Detailed Breakdown
- Step 1: Dehydrohalogenation to Propene
- Step 2: Hydroboration-Oxidation to 1-Propanol
- Step 3: Conversion of 1-Propanol to 1-Chloropropane
- Step 4: Nucleophilic Substitution to 1-Bromopropane
- Alternative Approaches and Considerations
- Potential Challenges and Troubleshooting
- Conclusion: A Strategic Synthesis
- Latest Posts
- Latest Posts
- Related Post
Devising a 4-Step Synthesis of 1-Bromopropane from 2-Bromopropane
The conversion of 2-bromopropane to 1-bromopropane might seem like a straightforward task, but it requires a strategic approach involving multiple steps. A direct substitution is not feasible due to the steric hindrance around the secondary carbon in 2-bromopropane. This article details a four-step synthesis, explaining the rationale behind each step, the reagents used, and the mechanisms involved. We'll also delve into potential challenges and explore alternative approaches.
Understanding the Challenge: Steric Hindrance and Regioselectivity
The key obstacle in directly converting 2-bromopropane to 1-bromopropane lies in regioselectivity. Nucleophilic substitution reactions (SN1 and SN2) are influenced by steric factors. The secondary carbon in 2-bromopropane is sterically hindered, making a direct SN2 substitution to form 1-bromopropane difficult and inefficient. An SN1 reaction, while less hindered, would still favor the more substituted carbocation intermediate, leading primarily to the reformation of 2-bromopropane. Therefore, a multi-step approach is necessary.
The Four-Step Synthesis: A Detailed Breakdown
Our four-step synthesis cleverly circumvents the steric hindrance problem by exploiting the reactivity of alkenes and the regioselectivity of hydrobromination.
Step 1: Dehydrohalogenation to Propene
The first step involves eliminating HBr from 2-bromopropane to form propene. This is an elimination reaction, specifically a dehydrohalogenation, commonly achieved using a strong base.
- Reagent: A strong base like potassium tert-butoxide (t-BuOK) in an aprotic solvent (like tert-butanol) is ideal. Strong bases are needed to abstract a proton, and an aprotic solvent prevents competing reactions.
- Mechanism: This is typically an E2 mechanism, where the base abstracts a proton anti-periplanar to the bromine, leading to the simultaneous formation of the double bond and expulsion of bromide ion. This is a concerted process.
- Product: Propene (CH3CH=CH2). Saytzeff's rule predicts the more substituted alkene (propene) will be the major product.
Step 2: Hydroboration-Oxidation to 1-Propanol
The next step converts propene into 1-propanol, effectively adding an OH group to the less substituted carbon. This utilizes hydroboration-oxidation, a powerful and regioselective method for anti-Markovnikov addition of water across a double bond.
- Reagents: Borane (BH3), usually as a complex like BH3·THF (borane-tetrahydrofuran complex), followed by an oxidizing agent like hydrogen peroxide (H2O2) in the presence of a base (like NaOH).
- Mechanism: Borane adds to the double bond in a syn fashion, with the boron attaching to the less hindered carbon (anti-Markovnikov addition). Oxidation then replaces the boron with a hydroxyl group.
- Product: 1-Propanol (CH3CH2CH2OH). The hydroboration-oxidation sequence ensures the hydroxyl group is placed on the terminal carbon.
Step 3: Conversion of 1-Propanol to 1-Chloropropane
This step converts the alcohol into a better leaving group for the final bromination step. Chlorination is favored over direct bromination as it allows for better control in the final step.
- Reagents: Thionyl chloride (SOCl2) is frequently used.
- Mechanism: SOCl2 reacts with the alcohol via an SN2 mechanism, replacing the hydroxyl group with a chlorine atom. This forms 1-chloropropane and produces sulfur dioxide (SO2) and HCl as byproducts.
- Product: 1-Chloropropane (CH3CH2CH2Cl).
Step 4: Nucleophilic Substitution to 1-Bromopropane
The final step involves substituting the chlorine atom in 1-chloropropane with a bromine atom through a nucleophilic substitution reaction.
- Reagent: Sodium bromide (NaBr) in a polar aprotic solvent like acetone is suitable.
- Mechanism: This is an SN2 reaction where the bromide ion acts as a nucleophile, attacking the carbon atom bonded to the chlorine, leading to the displacement of chloride ion. The polar aprotic solvent helps to stabilize the transition state.
- Product: 1-Bromopropane (CH3CH2CH2Br).
Alternative Approaches and Considerations
While the four-step synthesis detailed above is a reliable method, alternative approaches exist. These might involve different reagents or reaction sequences. For instance, you could potentially explore using a different elimination reaction in step 1, or using a different method to introduce the hydroxyl group in step 2. However, the hydroboration-oxidation method offers excellent regioselectivity for the desired anti-Markovnikov addition.
Potential Challenges and Troubleshooting
Several factors can influence the success of this synthesis:
- Purity of reagents: Impurities can significantly impact reaction yields and product purity. Using high-purity reagents is essential.
- Reaction conditions: Careful control of temperature, reaction time, and solvent is necessary for optimal results. Deviations from the optimal conditions may lead to reduced yields or the formation of side products.
- Work-up procedures: Proper work-up procedures, including extraction, washing, and drying, are vital for isolating the desired products and removing impurities.
Conclusion: A Strategic Synthesis
The four-step synthesis presented here demonstrates a practical and efficient method for converting 2-bromopropane to 1-bromopropane. By strategically employing elimination, hydroboration-oxidation, and nucleophilic substitution reactions, the steric hindrance issue associated with direct substitution is overcome. While alternative routes might exist, this method provides a clear, well-understood pathway with good yields, emphasizing the importance of planning synthetic strategies to achieve specific regiochemical outcomes. Careful consideration of reaction conditions and proper execution of each step are crucial for successful completion. Understanding the mechanisms involved in each step allows for troubleshooting and optimization, ultimately leading to higher yields and purer products. Remember to always prioritize safety and adhere to proper laboratory procedures when conducting chemical syntheses.
Latest Posts
Latest Posts
-
Liquid Sodium Is Being Considered As An Engine Coolant
Mar 31, 2025
-
A Runner Runs Around The Track At A Constant Speed
Mar 31, 2025
-
Cause Of Doody Out Of Business
Mar 31, 2025
-
You Should In Order To Document Data Properly
Mar 31, 2025
-
Which Statement Accurately Describes Type 2 Diabetes
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Devise A 4-step Synthesis Of 1-bromopropane From 2-bromopropane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
