Draw The Product Of The E2 Reaction Shown Below
Holbox
Mar 24, 2025 · 6 min read
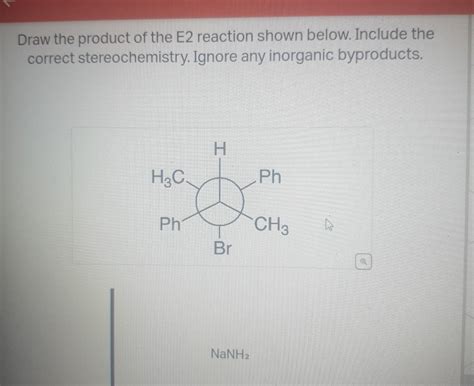
Table of Contents
- Draw The Product Of The E2 Reaction Shown Below
- Table of Contents
- Drawing the Product of an E2 Reaction: A Comprehensive Guide
- Understanding the E2 Reaction Mechanism
- Key Features of the E2 Reaction:
- Predicting the Product: A Step-by-Step Approach
- Examples and Detailed Explanations
- Advanced Considerations
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Drawing the Product of an E2 Reaction: A Comprehensive Guide
Elimination reactions, particularly E2 reactions, are a cornerstone of organic chemistry. Understanding how to predict the product of an E2 reaction is crucial for success in organic chemistry courses and beyond. This comprehensive guide will delve into the intricacies of E2 reactions, providing a step-by-step approach to accurately drawing the product, considering stereochemistry and regioselectivity. We'll explore various examples and address common challenges faced by students.
Understanding the E2 Reaction Mechanism
The E2 (bimolecular elimination) reaction is a single-step process where a base abstracts a proton (H+) from a β-carbon (the carbon adjacent to the carbon bearing the leaving group) while simultaneously eliminating the leaving group. This concerted mechanism necessitates a specific arrangement of atoms known as anti-periplanar geometry.
Key Features of the E2 Reaction:
- Concerted Mechanism: The proton abstraction and leaving group departure occur simultaneously in one step.
- Strong Base: A strong base, such as potassium tert-butoxide (t-BuOK), sodium ethoxide (NaOEt), or sodium hydroxide (NaOH), is required. Weak bases are generally ineffective.
- Anti-Periplanar Geometry: The proton and the leaving group must be anti-periplanar, meaning they are on opposite sides of the molecule and in the same plane. This geometric requirement is critical for the reaction to proceed.
- Steric Hindrance: Steric hindrance can influence the outcome of the reaction, favoring the formation of less substituted alkenes in some cases.
- Regioselectivity: In cases with multiple possible β-hydrogens, the reaction may show regioselectivity, preferring the formation of a more substituted alkene (Zaitsev's rule) or a less substituted alkene (Hofmann's rule).
Predicting the Product: A Step-by-Step Approach
Let's break down the process of predicting the product of an E2 reaction step-by-step, using examples to illustrate each point.
Step 1: Identify the Substrate and Base
Carefully examine the starting material (substrate) and the base used in the reaction. The substrate contains a leaving group (LG) and at least one β-hydrogen. The strong base will abstract the β-hydrogen.
Step 2: Locate the β-Hydrogens
Identify all the β-carbons (carbons adjacent to the carbon bearing the leaving group) and the hydrogens attached to them (β-hydrogens). These are the potential sites for proton abstraction.
Step 3: Consider Anti-Periplanar Geometry
This is the most critical step. The β-hydrogen and the leaving group must be anti-periplanar for the E2 reaction to occur. This means they need to be 180 degrees apart. You'll need to consider different conformations of the molecule to find the one that satisfies this requirement. Newman projections are often helpful in visualizing this geometry.
Step 4: Draw the Transition State
While not always explicitly drawn, visualizing the transition state can aid in predicting the product. The transition state involves the simultaneous breaking of the C-H and C-LG bonds and the formation of the π-bond.
Step 5: Determine the Product Alkene
Once you’ve identified the anti-periplanar β-hydrogen and the leaving group, remove the β-hydrogen and the leaving group, forming a double bond between the α-carbon (carbon with the leaving group) and the β-carbon.
Step 6: Account for Regioselectivity (Zaitsev's and Hofmann's Rules)
-
Zaitsev's Rule: Generally, the E2 reaction favors the formation of the more substituted alkene (the alkene with more alkyl groups attached to the double bond). This is the thermodynamically more stable product.
-
Hofmann's Rule: In certain cases, particularly with bulky bases or hindered substrates, the less substituted alkene (the alkene with fewer alkyl groups attached to the double bond) is the major product. This is often kinetically controlled.
Step 7: Consider Stereochemistry
The stereochemistry of the starting material significantly impacts the stereochemistry of the product. The E2 reaction leads to the formation of either an E or Z alkene depending on the stereochemistry of the starting material and which β-hydrogen is abstracted. Remember that E indicates the higher priority groups are on opposite sides of the double bond, while Z indicates they are on the same side.
Examples and Detailed Explanations
Let's work through some examples to solidify our understanding.
Example 1: A Simple E2 Reaction
Let's consider the reaction of 2-bromobutane with potassium tert-butoxide (t-BuOK).
(Image would be inserted here showing the reaction of 2-bromobutane with t-BuOK, leading to the formation of 2-butene. The anti-periplanar arrangement should be clearly illustrated.)
In this case, the t-BuOK abstracts a β-hydrogen, and the bromine atom departs, forming 2-butene. Since there are two possible β-hydrogens (one on each side of the molecule), both cis and trans 2-butene will form, with the trans isomer being the major product (due to Zaitsev's rule).
Example 2: Illustrating Anti-Periplanar Geometry and Stereochemistry
Consider the reaction of a chiral molecule, for example, a substituted cyclohexane, reacting with a strong base. Determining the product requires careful consideration of the molecule's conformation.
(Image would be inserted here showing a chiral molecule undergoing E2 reaction. The illustration should highlight the importance of the anti-periplanar geometry and demonstrate how different conformations lead to different products.)
The key here is to draw different conformations of the starting material and identify the conformations where a β-hydrogen and the leaving group are anti-periplanar. Only these conformations will lead to an E2 reaction. The resulting alkene will have a specific stereochemistry.
Example 3: Influence of Steric Hindrance
Bulky bases and sterically hindered substrates can favor the formation of the less substituted alkene (Hofmann's rule).
(Image would be inserted here showing an example of an E2 reaction where Hofmann's rule is observed. A bulky base and a sterically hindered substrate should be illustrated.)
The bulky base prefers to abstract the less hindered β-hydrogen, leading to the formation of the less substituted alkene.
Example 4: Dealing with Multiple β-Hydrogens
When a substrate has multiple β-hydrogens, you need to consider all possible conformations and apply Zaitsev's or Hofmann's rule to predict the major and minor products.
(Image would be inserted here showing a molecule with multiple β-hydrogens. The reaction should demonstrate the formation of different alkenes, with the major product indicated according to Zaitsev's rule.)
Carefully examine the possible anti-periplanar arrangements and assess the stability of the resulting alkenes. The more substituted alkene (Zaitsev's product) is generally favored, unless steric hindrance or a bulky base dictates otherwise (Hofmann's product).
Advanced Considerations
- Competition with SN2 Reactions: Primary alkyl halides often undergo SN2 reactions instead of E2 reactions, especially with weaker bases.
- Solvent Effects: The choice of solvent can influence the rate and selectivity of the E2 reaction. Polar aprotic solvents generally favor E2 reactions.
- Leaving Group Ability: The nature of the leaving group affects the reaction rate. Better leaving groups (e.g., tosylate, bromide, iodide) lead to faster reactions.
Conclusion
Predicting the product of an E2 reaction requires a thorough understanding of the reaction mechanism, including anti-periplanar geometry, regioselectivity, and stereochemistry. By systematically following the steps outlined in this guide and practicing with various examples, you can confidently draw the products of even complex E2 reactions. Remember to consider all possible conformations, identify the anti-periplanar relationships, and apply Zaitsev's or Hofmann's rule as appropriate. Mastering E2 reactions is a significant step toward a deeper understanding of organic chemistry.
Latest Posts
Latest Posts
-
A Clean Cut Of Leadership Quizlet
Mar 27, 2025
-
Indicate A Mild And Environmentally Safe Oxidizing Agent S
Mar 27, 2025
-
Simultaneously Insert Two Blank Rows Above Row 17
Mar 27, 2025
-
The Pth Percentile Is A Value Such That Approximately
Mar 27, 2025
-
Where Are You Allowed To Wash Your Hands
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Draw The Product Of The E2 Reaction Shown Below . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
