Label The Different Parts Of The Nucleotide Below
Holbox
Mar 21, 2025 · 6 min read
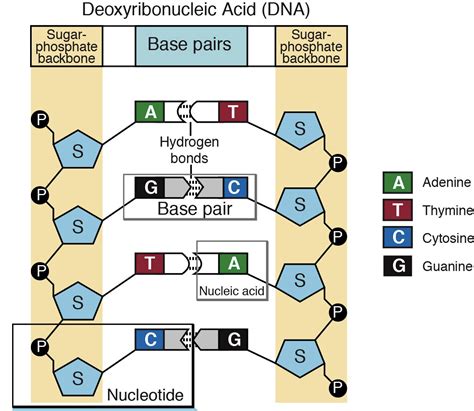
Table of Contents
- Label The Different Parts Of The Nucleotide Below
- Table of Contents
- Label the Different Parts of the Nucleotide Below: A Deep Dive into Nucleic Acid Structure
- The Core Components of a Nucleotide: A Tripartite Structure
- Detailed Examination of Each Component
- Nucleotide Nomenclature and Representation
- Nucleotides and Nucleic Acid Formation: From Monomer to Polymer
- The Significance of Nucleotide Structure in Biological Processes
- Conclusion: A Foundation of Life
- Latest Posts
- Latest Posts
- Related Post
Label the Different Parts of the Nucleotide Below: A Deep Dive into Nucleic Acid Structure
Nucleotides are the fundamental building blocks of nucleic acids, the molecules that carry genetic information in all living organisms. Understanding their structure is crucial to grasping the complexities of DNA and RNA. This article provides a comprehensive guide to identifying and understanding the different components of a nucleotide, exploring their chemical properties and their roles in the larger context of genetic information storage and transfer.
The Core Components of a Nucleotide: A Tripartite Structure
A nucleotide is essentially a tripartite molecule composed of three key components:
-
A Nitrogenous Base: This is a cyclic organic molecule containing nitrogen atoms. There are five main types of nitrogenous bases found in DNA and RNA: adenine (A), guanine (G), cytosine (C), thymine (T), and uracil (U). Adenine and guanine are purines, characterized by a double-ring structure, while cytosine, thymine, and uracil are pyrimidines, possessing a single-ring structure. The specific nitrogenous base present determines the nucleotide's identity and plays a crucial role in base pairing during DNA replication and transcription.
-
A Pentose Sugar: This is a five-carbon sugar molecule. In DNA, the pentose sugar is deoxyribose, while in RNA, it's ribose. The difference lies in the presence of a hydroxyl (-OH) group on the 2' carbon of ribose, which is absent in deoxyribose. This seemingly minor structural difference has significant implications for the stability and reactivity of DNA and RNA. Deoxyribose's lack of a 2'-OH group contributes to DNA's greater stability, making it a suitable molecule for long-term genetic information storage.
-
A Phosphate Group: This is a negatively charged molecule consisting of a phosphorus atom bonded to four oxygen atoms. The phosphate group connects to the 5' carbon of the pentose sugar, forming a phosphodiester bond with the adjacent nucleotide in a polynucleotide chain. The phosphate backbone is crucial for the overall structure and stability of nucleic acids. The negative charge on the phosphate group contributes to the hydrophilic nature of DNA and RNA, enabling their interaction with water and other cellular components.
Detailed Examination of Each Component
Let's delve deeper into the intricacies of each component:
1. Nitrogenous Bases: The Alphabet of Life
The nitrogenous bases are responsible for the variability within nucleic acids. The specific sequence of these bases dictates the genetic information encoded within DNA and RNA.
-
Purines (Adenine and Guanine): These double-ringed structures are relatively larger than pyrimidines. Their chemical properties, specifically the hydrogen-bonding potential of specific atoms within their ring structures, are crucial for base pairing. Adenine forms two hydrogen bonds with thymine (in DNA) or uracil (in RNA), while guanine forms three hydrogen bonds with cytosine.
-
Pyrimidines (Cytosine, Thymine, and Uracil): These single-ringed structures are smaller and less complex than purines. Cytosine is found in both DNA and RNA, while thymine is specific to DNA, and uracil is unique to RNA. The differences in their chemical structures directly impact base pairing specificity and the overall structure of the nucleic acid. The presence of methyl group in thymine (compared to uracil) also plays a role in DNA stability and protection against mutations.
2. Pentose Sugars: The Backbone's Foundation
The pentose sugar provides the structural framework to which the nitrogenous base and phosphate group attach. The subtle differences between deoxyribose and ribose significantly influence the properties of DNA and RNA.
-
Deoxyribose (in DNA): The absence of the hydroxyl group on the 2' carbon makes DNA more stable and resistant to hydrolysis, a process that breaks down the molecule. This stability is crucial for long-term storage of genetic information. The absence of this hydroxyl group also affects the conformation of the DNA double helix.
-
Ribose (in RNA): The presence of the hydroxyl group on the 2' carbon makes RNA more reactive and less stable than DNA. This increased reactivity makes RNA more susceptible to degradation, but also allows it to participate in a wider range of catalytic and regulatory roles within the cell.
3. Phosphate Groups: The Connecting Link
The phosphate groups link adjacent nucleotides in a polynucleotide chain, creating a strong and negatively charged backbone.
-
Phosphodiester Bonds: The phosphate group forms phosphodiester bonds between the 3' carbon of one pentose sugar and the 5' carbon of the next. This creates a directional chain with a 5' end (phosphate group attached to the 5' carbon) and a 3' end (hydroxyl group on the 3' carbon). This directionality is essential for DNA replication and transcription.
-
Negative Charge: The negative charge of the phosphate backbone contributes to the hydrophilic nature of DNA and RNA, enabling their interaction with water and various proteins involved in DNA replication, transcription, and repair. This negative charge also plays a role in the electrostatic interactions within the DNA double helix.
Nucleotide Nomenclature and Representation
Nucleotides are often represented using abbreviations that reflect their components. For example:
- dAMP: deoxyadenosine monophosphate (a nucleotide containing deoxyribose, adenine, and one phosphate group)
- dGMP: deoxyguanosine monophosphate
- dCMP: deoxycytidine monophosphate
- dTMP: deoxythymidine monophosphate
- AMP: adenosine monophosphate (a nucleotide containing ribose, adenine, and one phosphate group)
- GMP: guanosine monophosphate
- CMP: cytidine monophosphate
- UMP: uridine monophosphate
The "d" prefix indicates deoxyribose, while the absence of "d" indicates ribose. The suffix "-mono," "-di," or "-tri" phosphate indicates the number of phosphate groups attached. Nucleotides with more than one phosphate group (diphosphate or triphosphate) are crucial energy carriers (like ATP) and play a vital role in metabolic processes.
Nucleotides and Nucleic Acid Formation: From Monomer to Polymer
Nucleotides are the monomers that polymerize to form nucleic acids, DNA and RNA. The polymerization process involves the formation of phosphodiester bonds between the 3' hydroxyl group of one nucleotide and the 5' phosphate group of the next. This process continues, creating a long chain of nucleotides linked together by phosphodiester bonds.
The Significance of Nucleotide Structure in Biological Processes
The precise structure of nucleotides is fundamental to many essential biological processes. Understanding their structure is key to understanding:
- DNA Replication: The specific base pairing (A-T and G-C in DNA) is crucial for accurate replication of genetic information.
- Transcription: The process of creating RNA from a DNA template relies on base pairing between the DNA and the newly synthesized RNA molecule.
- Translation: The sequence of nucleotides in mRNA determines the sequence of amino acids in proteins.
- Energy Transfer: ATP (adenosine triphosphate) and other nucleotides with multiple phosphate groups are vital energy carriers in cellular metabolism.
- Signal Transduction: Cyclic nucleotides, such as cAMP (cyclic adenosine monophosphate), act as second messengers in various signal transduction pathways.
- Enzyme Regulation: Some nucleotides play a crucial role in regulating the activity of enzymes.
Conclusion: A Foundation of Life
The seemingly simple structure of a nucleotide belies its profound importance in all living organisms. The careful arrangement of its three components – nitrogenous base, pentose sugar, and phosphate group – underpins the structure and function of DNA and RNA, the molecules that govern the inheritance, expression, and regulation of genetic information. A thorough understanding of nucleotide structure is a cornerstone of modern biology and crucial for advancements in areas such as medicine, biotechnology, and genetic engineering. Further research continues to unravel the complexities of nucleotide function and its implications for a wide range of biological processes.
Latest Posts
Latest Posts
-
Regular Inspections Performed In Facilities Are Called
Mar 23, 2025
-
Construct A Multistep Synthetic Route From Ethylbenzene
Mar 23, 2025
-
Pn Management Online Practice 2023 B
Mar 23, 2025
-
This Country Restricts Foreign Investment In Tobacco And Mining
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Label The Different Parts Of The Nucleotide Below . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
