Construct A Multistep Synthetic Route From Ethylbenzene
Holbox
Mar 23, 2025 · 6 min read
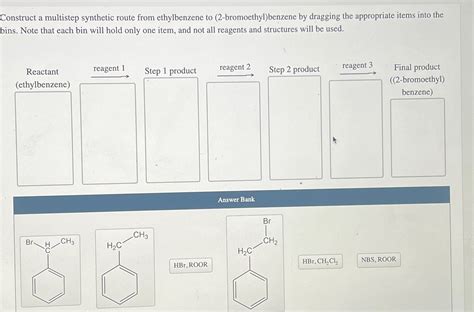
Table of Contents
- Construct A Multistep Synthetic Route From Ethylbenzene
- Table of Contents
- Constructing a Multi-Step Synthetic Route from Ethylbenzene: A Comprehensive Guide
- Understanding Ethylbenzene's Reactivity
- Synthetic Route 1: Synthesis of Acetophenone
- Step 1: Oxidation of Ethylbenzene to Phenylacetic Acid
- Step 2: Decarboxylation of Phenylacetic Acid to Acetophenone
- Synthetic Route 2: Synthesis of Styrene
- Step 1: Dehydrogenation of Ethylbenzene
- Synthetic Route 3: Synthesis of Benzyl Chloride and Benzyl Alcohol
- Step 1: Free Radical Chlorination of Ethylbenzene
- Step 2: Hydrolysis of Benzyl Chloride to Benzyl Alcohol
- Synthetic Route 4: Synthesis of Nitro-substituted Ethylbenzenes
- Step 1: Nitration of Ethylbenzene
- Optimizing Synthetic Routes
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Constructing a Multi-Step Synthetic Route from Ethylbenzene: A Comprehensive Guide
Ethylbenzene, a simple aromatic hydrocarbon, serves as a versatile starting material for a vast array of organic compounds. Its reactivity, stemming from the presence of both an alkyl and an aryl group, allows for a diverse range of transformations, leading to complex molecules with significant industrial and pharmaceutical applications. This article delves into the construction of multi-step synthetic routes starting from ethylbenzene, highlighting key reactions, reaction mechanisms, and considerations for optimizing yield and selectivity. We'll explore several different pathways, showcasing the versatility of ethylbenzene as a building block.
Understanding Ethylbenzene's Reactivity
Before embarking on synthetic routes, it's crucial to understand ethylbenzene's reactivity profile. The presence of the ethyl group activates the benzene ring towards electrophilic aromatic substitution, while the alkyl chain itself is susceptible to oxidation, halogenation, and other reactions. This dual reactivity allows for a rich tapestry of synthetic possibilities. The key reactions we will leverage include:
-
Electrophilic Aromatic Substitution: This is a cornerstone reaction, allowing for the introduction of various functional groups onto the benzene ring. Common electrophiles include halogens (chlorine, bromine), nitro groups, and sulfonic acid groups. The presence of the ethyl group influences the regioselectivity, directing substituents to the ortho and para positions.
-
Oxidation of the Alkyl Chain: The ethyl group can be oxidized to a carboxylic acid group, providing a route to aromatic acids. This oxidation typically employs strong oxidizing agents like potassium permanganate (KMnO₄) or chromic acid (H₂CrO₄).
-
Halogenation of the Alkyl Chain: The alkyl chain can undergo free radical halogenation, introducing halogen atoms at the benzylic position (the carbon atom directly attached to the benzene ring). This is usually achieved using halogen molecules (Cl₂, Br₂) and a free radical initiator, such as light or peroxides.
-
Dehydrogenation: Under specific conditions, ethylbenzene can undergo dehydrogenation to form styrene, a valuable monomer for polymer production. This often requires high temperatures and catalysts.
Synthetic Route 1: Synthesis of Acetophenone
One common target molecule synthesized from ethylbenzene is acetophenone. This involves a two-step process:
Step 1: Oxidation of Ethylbenzene to Phenylacetic Acid
Ethylbenzene can be oxidized to phenylacetic acid using strong oxidizing agents such as potassium permanganate (KMnO₄) in an alkaline solution. The reaction proceeds via a series of intermediate steps, eventually cleaving the carbon-carbon bond of the ethyl group to form the carboxylic acid.
Reaction:
Ethylbenzene + KMnO₄ (alkaline) → Phenylacetic acid
Mechanism: This involves a complex series of redox reactions. The permanganate ion acts as an oxidant, abstracting electrons from the alkyl chain. This leads to the formation of a carboxylate anion, which is then protonated to yield phenylacetic acid. Careful control of reaction conditions is crucial to maximize the yield and minimize side reactions.
Step 2: Decarboxylation of Phenylacetic Acid to Acetophenone
Phenylacetic acid can be decarboxylated to acetophenone using a variety of methods. One common approach involves heating phenylacetic acid in the presence of a strong acid catalyst, such as sulfuric acid (H₂SO₄). This reaction involves the loss of carbon dioxide (CO₂) and the formation of a ketone.
Reaction:
Phenylacetic acid + H₂SO₄ (heat) → Acetophenone + CO₂
Mechanism: This involves the protonation of the carboxylic acid group, followed by the loss of CO₂ to form a carbocation intermediate. This intermediate then undergoes rearrangement and deprotonation to yield acetophenone.
Synthetic Route 2: Synthesis of Styrene
Styrene, a crucial monomer in the production of polystyrene and other polymers, can be synthesized from ethylbenzene through a dehydrogenation process.
Step 1: Dehydrogenation of Ethylbenzene
Ethylbenzene undergoes dehydrogenation at high temperatures (typically above 600°C) in the presence of a suitable catalyst, such as iron oxide (Fe₂O₃) or zinc oxide (ZnO). This process involves the removal of two hydrogen atoms from the ethyl group, forming a carbon-carbon double bond and yielding styrene.
Reaction:
Ethylbenzene (heat, catalyst) → Styrene + H₂
Mechanism: The catalyst facilitates the removal of hydrogen atoms, likely through a series of adsorption and desorption steps on the catalyst surface. The precise mechanism is complex and depends on the specific catalyst employed.
Synthetic Route 3: Synthesis of Benzyl Chloride and Benzyl Alcohol
These compounds are valuable intermediates in organic synthesis. Their synthesis from ethylbenzene involves free radical halogenation and subsequent substitution reactions.
Step 1: Free Radical Chlorination of Ethylbenzene
Free radical chlorination of ethylbenzene selectively introduces a chlorine atom at the benzylic position, yielding benzyl chloride. This reaction is typically carried out using chlorine gas (Cl₂) in the presence of a free radical initiator, such as ultraviolet (UV) light or peroxides.
Reaction:
Ethylbenzene + Cl₂ (UV light) → Benzyl chloride + HCl
Mechanism: This involves a chain reaction mechanism. The initiator generates chlorine radicals, which abstract a hydrogen atom from the benzylic position of ethylbenzene, forming a benzyl radical. The benzyl radical then reacts with a chlorine molecule to form benzyl chloride and another chlorine radical, propagating the chain reaction.
Step 2: Hydrolysis of Benzyl Chloride to Benzyl Alcohol
Benzyl chloride can be converted to benzyl alcohol via hydrolysis. This reaction is typically carried out by treating benzyl chloride with aqueous sodium hydroxide (NaOH) or potassium hydroxide (KOH).
Reaction:
Benzyl chloride + NaOH (aqueous) → Benzyl alcohol + NaCl
Mechanism: This involves a nucleophilic substitution reaction. The hydroxide ion (OH⁻) acts as a nucleophile, attacking the benzylic carbon atom and displacing the chloride ion.
Synthetic Route 4: Synthesis of Nitro-substituted Ethylbenzenes
Introducing nitro groups onto the benzene ring of ethylbenzene can yield valuable intermediates for further transformations.
Step 1: Nitration of Ethylbenzene
Ethylbenzene undergoes nitration when treated with a mixture of concentrated nitric acid (HNO₃) and concentrated sulfuric acid (H₂SO₄). This reaction introduces a nitro group (-NO₂) onto the benzene ring. The ethyl group directs the nitration primarily to the ortho and para positions.
Reaction:
Ethylbenzene + HNO₃/H₂SO₄ → ortho-Nitroethylbenzene + para-Nitroethylbenzene
Mechanism: This is a classic electrophilic aromatic substitution. The nitronium ion (NO₂⁺), generated from the mixture of nitric and sulfuric acids, acts as the electrophile, attacking the benzene ring.
Optimizing Synthetic Routes
Optimizing yield and selectivity in these multi-step syntheses is crucial for efficient production. Several factors influence the outcome:
-
Reaction Conditions: Temperature, pressure, and concentration of reactants significantly impact reaction rates and selectivity. Careful control of these parameters is essential.
-
Catalyst Selection: The choice of catalyst can significantly influence reaction rate, selectivity, and yield. Careful consideration of catalyst properties is crucial.
-
Purification Techniques: Effective purification techniques, such as distillation, recrystallization, or chromatography, are essential to isolate the desired product and remove impurities.
-
Reaction Monitoring: Monitoring the progress of the reaction using techniques like gas chromatography (GC) or high-performance liquid chromatography (HPLC) allows for real-time optimization and adjustment of reaction conditions.
Conclusion
Ethylbenzene, despite its seemingly simple structure, serves as a surprisingly versatile building block for the synthesis of a wide range of important organic compounds. By strategically employing various reaction types, such as electrophilic aromatic substitution, oxidation, halogenation, and dehydrogenation, chemists can access a diverse array of molecules with significant industrial and pharmaceutical applications. Careful optimization of reaction conditions and purification techniques is crucial for maximizing yield and selectivity in these multi-step synthetic routes. The examples provided in this article only scratch the surface of the synthetic potential of ethylbenzene, offering a starting point for further exploration and innovation in organic synthesis. Understanding the underlying reaction mechanisms and mastering the control of reaction conditions are paramount for success in constructing efficient and high-yielding synthetic pathways.
Latest Posts
Latest Posts
-
Draw The Correct Organic Product For The Oxidation Reaction
Mar 25, 2025
-
What Are Twitters Most Important Assets
Mar 25, 2025
-
The Term Institutionalization Can Be Defined As
Mar 25, 2025
-
Complete The Synthetic Division Problem Below 2 1 5
Mar 25, 2025
-
Ohrp Is An Oversight Body Primarily Concerned With
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Construct A Multistep Synthetic Route From Ethylbenzene . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
