Draw The Correct Organic Product For The Oxidation Reaction
Holbox
Mar 25, 2025 · 6 min read
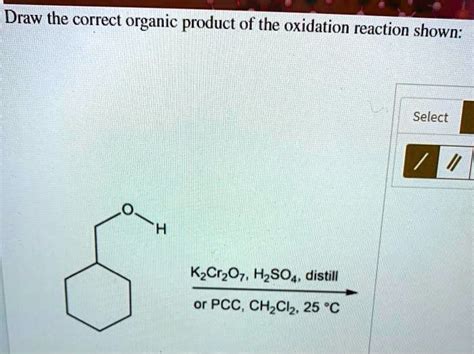
Table of Contents
- Draw The Correct Organic Product For The Oxidation Reaction
- Table of Contents
- Drawing the Correct Organic Product for Oxidation Reactions: A Comprehensive Guide
- Understanding Oxidation in Organic Chemistry
- Common Oxidizing Agents and Their Selectivity
- 1. Potassium Permanganate (KMnO₄)
- 2. Jones Reagent (CrO₃/H₂SO₄)
- 3. PCC (Pyridinium Chlorochromate)
- 4. Swern Oxidation
- 5. Osmium Tetroxide (OsO₄)
- 6. Ozone (O₃) followed by a Reducing Agent (e.g., Zn, DMS)
- Predicting Products: A Step-by-Step Approach
- Advanced Considerations and Challenges
- Case Studies: Illustrative Examples
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Drawing the Correct Organic Product for Oxidation Reactions: A Comprehensive Guide
Oxidation reactions are a cornerstone of organic chemistry, transforming molecules by increasing their oxidation state. Mastering the prediction of products in these reactions is crucial for success in organic chemistry. This comprehensive guide will delve into the intricacies of oxidation reactions, focusing on how to accurately predict the organic products formed. We’ll explore various oxidizing agents, their selectivity, and the specific reactions they catalyze, equipping you with the tools to confidently tackle even the most complex oxidation problems.
Understanding Oxidation in Organic Chemistry
Before we dive into specific reactions, it's vital to understand the concept of oxidation in the context of organic molecules. In organic chemistry, oxidation is often defined as an increase in the number of bonds to oxygen or a decrease in the number of bonds to hydrogen. This can manifest in several ways:
-
Alcohol Oxidation: Primary alcohols (R-CH₂OH) can be oxidized to aldehydes (R-CHO) and further to carboxylic acids (R-COOH). Secondary alcohols (R₂CHOH) are oxidized to ketones (R₂C=O). Tertiary alcohols (R₃COH) are resistant to oxidation due to the lack of a hydrogen atom on the carbon bearing the hydroxyl group.
-
Aldehyde Oxidation: Aldehydes are readily oxidized to carboxylic acids.
-
Alkene Oxidation: Alkenes can undergo various oxidation reactions, leading to the formation of epoxides, diols, or carbonyl compounds depending on the oxidizing agent used.
-
Alkane Oxidation: Alkanes, generally resistant to oxidation, can be oxidized under harsh conditions, often leading to the formation of alcohols or ketones.
Common Oxidizing Agents and Their Selectivity
The choice of oxidizing agent significantly impacts the outcome of an oxidation reaction. Different reagents possess varying strengths and selectivities. Let's examine some commonly used oxidizing agents:
1. Potassium Permanganate (KMnO₄)
KMnO₄ is a strong oxidizing agent capable of oxidizing primary alcohols to carboxylic acids and secondary alcohols to ketones. It's also effective in oxidizing alkenes to diols. The reaction conditions (acidic, basic, or neutral) can influence the final product. For instance, in acidic conditions, KMnO₄ can cleave vicinal diols.
Example: Oxidation of a primary alcohol using KMnO₄ in acidic conditions yields a carboxylic acid.
2. Jones Reagent (CrO₃/H₂SO₄)
Jones reagent is a powerful and versatile oxidizing agent primarily used for the oxidation of primary and secondary alcohols. Primary alcohols are oxidized to carboxylic acids, while secondary alcohols are oxidized to ketones. It's less commonly used for alkenes due to its tendency to over-oxidize.
Example: Oxidation of a secondary alcohol with Jones reagent results in the formation of a ketone.
3. PCC (Pyridinium Chlorochromate)
PCC is a milder oxidizing agent compared to KMnO₄ or Jones reagent. It selectively oxidizes primary alcohols to aldehydes without further oxidation to carboxylic acids. Secondary alcohols are oxidized to ketones.
Example: PCC oxidation of a primary alcohol stops at the aldehyde stage.
4. Swern Oxidation
The Swern oxidation employs dimethyl sulfoxide (DMSO) and oxalyl chloride to oxidize primary and secondary alcohols to aldehydes and ketones respectively. It's known for its mild conditions and its ability to handle sensitive functional groups.
Example: Swern oxidation is effective for oxidizing alcohols while minimizing side reactions.
5. Osmium Tetroxide (OsO₄)
OsO₄ is a powerful oxidizing agent used for the syn dihydroxylation of alkenes. This means that two hydroxyl groups are added to the alkene from the same side, resulting in a cis diol.
Example: OsO₄ converts an alkene to a cis diol.
6. Ozone (O₃) followed by a Reducing Agent (e.g., Zn, DMS)
Ozonolysis is a powerful method for cleaving alkenes. Ozone reacts with alkenes to form ozonides, which are subsequently reduced to aldehydes or ketones depending on the substitution pattern of the alkene.
Example: Ozonolysis of a symmetrical alkene yields two equivalents of the same aldehyde.
Predicting Products: A Step-by-Step Approach
To accurately predict the organic product of an oxidation reaction, follow these steps:
-
Identify the functional group: Determine the functional group present in the starting material (alcohol, aldehyde, alkene, etc.).
-
Choose the appropriate oxidizing agent: Select the oxidizing agent based on the desired transformation and the functional group present. Consider the strength and selectivity of the reagent.
-
Determine the oxidation state change: Analyze the change in the oxidation state of the carbon atom(s) involved in the reaction. Remember the definition of oxidation as an increase in bonds to oxygen or a decrease in bonds to hydrogen.
-
Draw the product: Based on the change in oxidation state and the selectivity of the oxidizing agent, draw the structure of the organic product. Be mindful of stereochemistry, especially in reactions involving alkenes or chiral centers.
-
Consider side reactions: Some oxidizing agents can lead to side reactions, especially under harsh conditions. Be aware of these possibilities when predicting the product.
Advanced Considerations and Challenges
Several advanced aspects of oxidation reactions require careful consideration:
-
Stereochemistry: Certain oxidations, particularly those involving alkenes, can influence the stereochemistry of the product. Pay close attention to syn vs. anti addition.
-
Over-oxidation: Strong oxidizing agents can lead to over-oxidation, resulting in unexpected products.
-
Protecting groups: In molecules with multiple functional groups, protecting groups may be necessary to prevent unwanted oxidation of specific functional groups.
-
Regioselectivity: Some oxidations exhibit regioselectivity, meaning that the oxidation occurs preferentially at a specific site within the molecule.
Case Studies: Illustrative Examples
Let's examine a few illustrative examples to solidify our understanding:
Example 1: Oxidation of 1-propanol
- Starting Material: 1-propanol (primary alcohol)
- Oxidizing Agent: KMnO₄ (acidic conditions)
- Product: Propanoic acid
Example 2: Oxidation of cyclohexanol
- Starting Material: Cyclohexanol (secondary alcohol)
- Oxidizing Agent: PCC
- Product: Cyclohexanone
Example 3: Ozonolysis of 2-butene
- Starting Material: 2-butene (alkene)
- Oxidizing Agent: O₃ followed by Zn
- Product: Two molecules of acetaldehyde
Example 4: Oxidation of benzaldehyde
- Starting Material: Benzaldehyde
- Oxidizing Agent: Ag₂O (Tollens' reagent)
- Product: Benzoic acid
These examples illustrate the diverse applications of oxidation reactions and the importance of selecting the appropriate oxidizing agent for a specific transformation.
Conclusion
Mastering the prediction of products in oxidation reactions requires a thorough understanding of the principles of oxidation, the properties of various oxidizing agents, and careful consideration of reaction conditions and potential side reactions. By following a systematic approach and practicing with diverse examples, you can build confidence in tackling complex oxidation problems and predicting the correct organic products with accuracy. Remember to always consider the specific oxidizing agent, its strength and selectivity, and the nature of the substrate to accurately predict the outcome of the reaction. This comprehensive guide provides a solid foundation for success in this vital area of organic chemistry. Continue practicing and exploring different examples to reinforce your understanding and build expertise in predicting the products of oxidation reactions.
Latest Posts
Latest Posts
-
Sam Is An It Manager A New Process
Mar 27, 2025
-
Costs That A Manager Can Determine Or Influence Are
Mar 27, 2025
-
A Simple Elevator Ride Can Teach
Mar 27, 2025
-
To Achieve The Profitable Distribution Of Exposures
Mar 27, 2025
-
A Camera Operator Is Filming A Nature Explorer
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Draw The Correct Organic Product For The Oxidation Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
