Is Honh3br An Acid Or Base
Holbox
Mar 26, 2025 · 5 min read
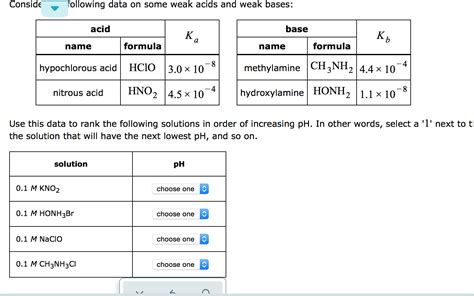
Table of Contents
- Is Honh3br An Acid Or Base
- Table of Contents
- Is HBr an Acid or a Base? Understanding the Properties of Hydrobromic Acid
- Understanding Acids and Bases: A Quick Refresher
- Arrhenius Theory: Focusing on H⁺ and OH⁻ Ions
- Brønsted-Lowry Theory: Proton Donors and Acceptors
- Lewis Theory: Electron Pair Donors and Acceptors
- Delving into Hydrobromic Acid (HBr): A Strong Acid
- Why is HBr a Strong Acid?
- Properties of Hydrobromic Acid: A Closer Look
- Applications of Hydrobromic Acid: Diverse Uses
- Safety Precautions when Handling Hydrobromic Acid
- Conclusion: HBr is Definitely an Acid
- Latest Posts
- Latest Posts
- Related Post
Is HBr an Acid or a Base? Understanding the Properties of Hydrobromic Acid
The question, "Is HBr an acid or a base?", might seem simple at first glance, but a deeper understanding requires exploring the fundamental concepts of acids and bases, along with the specific properties of hydrobromic acid (HBr). This comprehensive guide will delve into the chemical nature of HBr, its behavior in aqueous solutions, and its applications, ultimately providing a definitive answer to the central question.
Understanding Acids and Bases: A Quick Refresher
Before we dive into the specifics of HBr, let's briefly revisit the core concepts of acids and bases. Several theories exist to define these fundamental chemical entities, but the most common are the Arrhenius, Brønsted-Lowry, and Lewis theories.
Arrhenius Theory: Focusing on H⁺ and OH⁻ Ions
The Arrhenius theory, the oldest of the three, defines acids as substances that produce hydrogen ions (H⁺) when dissolved in water, while bases produce hydroxide ions (OH⁻). This theory is relatively simple but limited in its scope, as it doesn't encompass all acid-base reactions.
Brønsted-Lowry Theory: Proton Donors and Acceptors
The Brønsted-Lowry theory offers a broader perspective. It defines an acid as a proton donor, a molecule or ion that donates a proton (H⁺), and a base as a proton acceptor. This theory expands the definition to include reactions that don't necessarily involve water.
Lewis Theory: Electron Pair Donors and Acceptors
The Lewis theory, the most general of the three, defines acids as electron pair acceptors and bases as electron pair donors. This theory encompasses a wider range of reactions than the previous two, including those that don't involve proton transfer.
Delving into Hydrobromic Acid (HBr): A Strong Acid
Now, let's focus on hydrobromic acid (HBr). HBr is a strong acid, meaning it completely dissociates (ionizes) in water, releasing all its protons (H⁺) and bromide ions (Br⁻). This complete dissociation is a key characteristic distinguishing strong acids from weak acids, which only partially dissociate.
The reaction of HBr in water can be represented as follows:
HBr(aq) → H⁺(aq) + Br⁻(aq)
This equation clearly demonstrates that HBr donates a proton (H⁺) to water molecules, fitting the definition of an acid according to both the Arrhenius and Brønsted-Lowry theories. The complete dissociation further reinforces its classification as a strong acid. Furthermore, the H⁺ ion acts as an electron pair acceptor, fitting the Lewis definition of an acid as well.
Why is HBr a Strong Acid?
The strength of HBr as an acid stems from the relatively weak H-Br bond. The bond's weakness facilitates the easy release of the proton (H⁺), leading to complete dissociation in water. This contrasts with weaker acids like acetic acid (CH₃COOH), where the H-O bond is stronger, resulting in only partial dissociation.
Properties of Hydrobromic Acid: A Closer Look
HBr possesses several notable properties that contribute to its classification as a strong acid and its various applications:
- Highly Corrosive: HBr is extremely corrosive to many metals and tissues. It's essential to handle it with extreme caution, using appropriate personal protective equipment (PPE).
- Colorless Gas or Fuming Liquid: Pure HBr exists as a colorless gas, while its aqueous solutions are colorless or slightly yellowish fuming liquids.
- High Solubility in Water: HBr readily dissolves in water, forming highly acidic solutions.
- Pungent Odor: HBr has a pungent, irritating odor that makes detection crucial in the event of a leak.
- Excellent Solvent: Due to its acidic nature and high polarity, HBr can dissolve various substances.
- Strong Reducing Agent (in certain contexts): The bromide ion (Br⁻) can act as a reducing agent under specific conditions. This is not generally considered a dominant property in comparison to its acidic characteristics.
Applications of Hydrobromic Acid: Diverse Uses
The strong acidic nature and other properties of HBr make it useful in a wide variety of applications, including:
- Industrial Processes: HBr finds use in various industrial chemical syntheses, including the production of alkyl bromides, which are used as solvents and intermediates in pharmaceutical and agricultural chemical synthesis.
- Laboratory Reagent: In laboratories, HBr is a common reagent used in organic chemistry, for example, in the preparation of bromides and in some catalytic processes.
- Metal Refining: It can be used in metal processing and refining for dissolving specific metals.
- Etching: HBr can be used in the etching of certain materials.
- Petroleum Refining: It plays a minor role in certain petroleum refining processes.
Safety Precautions when Handling Hydrobromic Acid
Due to its highly corrosive and hazardous nature, strict safety precautions must be followed when working with HBr:
- Personal Protective Equipment (PPE): Always wear appropriate PPE, including chemical-resistant gloves, goggles, lab coats, and respiratory protection.
- Ventilation: Work in a well-ventilated area or use a fume hood to prevent inhalation of HBr fumes.
- Spill Response: Have a spill response plan in place, including neutralizing agents and proper cleanup procedures.
- Storage: Store HBr in a cool, dry place away from incompatible materials.
- First Aid: Be familiar with the first aid procedures for HBr exposure, including immediate flushing of affected areas with water.
Conclusion: HBr is Definitely an Acid
In conclusion, HBr is unequivocally classified as an acid. Its complete dissociation in water, releasing protons (H⁺), aligns perfectly with the Arrhenius and Brønsted-Lowry definitions of acids. Furthermore, its ability to act as an electron pair acceptor fits the Lewis definition as well. Its corrosive nature, widespread applications, and the need for careful handling further highlight its acidic character. The question, "Is HBr an acid or a base?" is answered definitively: HBr is a strong acid. Understanding its properties and handling it safely are crucial for anyone working with this important chemical reagent. This knowledge is essential for both industrial applications and academic research. Remember to always prioritize safety when working with HBr or any other strong acid.
Latest Posts
Latest Posts
-
Exercise 6 12 Bank Reconciliation Lo P3
Mar 29, 2025
-
Joe Decides To Build A Chicken Coop
Mar 29, 2025
-
A High Degree Of Operating Leverage Means
Mar 29, 2025
-
A Department That Incurs Costs Without Generating Revenues Is A
Mar 29, 2025
-
Find Y As A Function Of X If
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Is Honh3br An Acid Or Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
