Is Chlorine An Acid Or Base
Holbox
Mar 10, 2025 · 5 min read
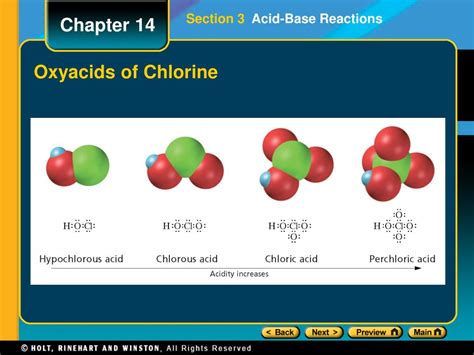
Table of Contents
Is Chlorine an Acid or a Base? Understanding Chlorine's Chemical Nature
Chlorine, a ubiquitous element with far-reaching applications, often sparks the question: is it an acid or a base? The answer isn't a simple "yes" or "no." Chlorine's acidic or basic nature depends heavily on its chemical form and the context in which it's encountered. This comprehensive article delves into the complexities of chlorine's behavior, exploring its various forms and their interactions with water to clarify its position within the acid-base spectrum.
Understanding Acids and Bases
Before diving into chlorine's nature, let's establish a clear understanding of acids and bases. Several theories define these fundamental concepts in chemistry. The most commonly used are:
Arrhenius Theory
The Arrhenius theory defines acids as substances that produce hydrogen ions (H⁺) when dissolved in water, and bases as substances that produce hydroxide ions (OH⁻) in water. This theory is straightforward but limited in scope, as it only applies to aqueous solutions.
Brønsted-Lowry Theory
The Brønsted-Lowry theory offers a broader definition. It defines acids as proton donors and bases as proton acceptors. This theory extends beyond aqueous solutions, encompassing reactions in other solvents or even gas-phase reactions.
Lewis Theory
The Lewis theory provides the most comprehensive definition. It defines acids as electron-pair acceptors and bases as electron-pair donors. This encompasses a wider range of reactions, including those not involving protons.
Chlorine's Chemical Forms and their Acid-Base Properties
Chlorine itself is a highly reactive nonmetal element existing as a diatomic molecule (Cl₂), a pale green gas at standard temperature and pressure. In its elemental form, chlorine isn't directly classified as an acid or a base according to the typical definitions. However, its behavior changes dramatically when it interacts with water or other substances.
Chlorine's Reaction with Water: Disproportionation
When chlorine gas (Cl₂) dissolves in water, it undergoes a disproportionation reaction. This means a single element is simultaneously oxidized and reduced, resulting in the formation of both a stronger oxidizing agent and a stronger reducing agent. The reaction is as follows:
Cl₂ + H₂O ⇌ HCl + HOCl
This reaction yields hydrochloric acid (HCl) and hypochlorous acid (HOCl). This is where the acidic nature of chlorine's interaction with water becomes apparent.
Hydrochloric Acid (HCl): A Strong Acid
Hydrochloric acid is a strong acid. It readily dissociates in water, releasing a high concentration of hydrogen ions (H⁺), significantly lowering the pH of the solution. This aligns perfectly with the Arrhenius and Brønsted-Lowry definitions of acids.
Hypochlorous Acid (HOCl): A Weak Acid
Hypochlorous acid, on the other hand, is a weak acid. While it does donate protons, it doesn't dissociate completely in water. This results in a lower concentration of H⁺ ions compared to HCl, making it a weaker acid.
Other Chlorine-Containing Compounds and their Acid-Base Properties
Beyond its reaction with water, chlorine forms numerous compounds exhibiting diverse acidic or basic properties. Here are some examples:
-
Sodium hypochlorite (NaClO): A Weak Base Often found in bleach, sodium hypochlorite is the sodium salt of hypochlorous acid. In solution, it hydrolyzes to produce hydroxide ions (OH⁻), exhibiting weak basic properties according to the Arrhenius definition.
-
Chlorine dioxide (ClO₂): Neither Acidic nor Basic This compound is a powerful oxidizing agent, but its behavior doesn't fit neatly into the acid-base framework.
-
Hydrogen chloride (HCl): A Strong Acid As discussed previously, this is a strong acid, completely dissociating in water to produce H⁺ ions.
-
Chlorates and Perchlorates: These are salts of chloric acid (HClO₃) and perchloric acid (HClO₄), respectively. Perchloric acid is considered one of the strongest acids known, demonstrating strong acidic character. The corresponding salts are generally neutral, although their solutions can exhibit weak acidic or basic properties depending on their counterions.
Factors Affecting Chlorine's Acid-Base Behavior
Several factors influence the extent to which chlorine behaves as an acid or a base:
-
Concentration: The concentration of chlorine in a solution will influence the equilibrium of the disproportionation reaction and the relative amounts of HCl and HOCl produced.
-
pH: The pH of the solution will affect the dissociation of HCl and HOCl. A lower pH will suppress the dissociation of both acids, while a higher pH will favor their dissociation.
-
Temperature: Temperature can also affect the equilibrium of the reactions and the degree of dissociation of the acids.
-
Presence of other chemicals: The presence of other chemicals in the solution can influence the reactions and the overall acidity or basicity of the system.
Practical Applications and Implications
The acidic or basic nature of chlorine and its compounds has numerous practical applications and implications:
-
Water treatment: Chlorine is widely used in water purification to kill harmful bacteria and viruses. The hypochlorous acid formed during the disinfection process is a crucial component of this process.
-
Bleach: Sodium hypochlorite is a common household bleaching agent, using its basic properties to lift stains and disinfect surfaces.
-
Industrial processes: Various chlorine compounds are used in numerous industrial applications, including the production of plastics, pharmaceuticals, and other chemicals. Understanding their acid-base properties is crucial for effective process control and safety.
-
Environmental considerations: Chlorine's impact on the environment needs careful consideration due to the potential formation of harmful byproducts in various reactions and applications.
Conclusion: The Nuances of Chlorine's Behavior
Chlorine's behavior isn't definitively classified as simply acidic or basic. Its nature is heavily context-dependent. While elemental chlorine isn't inherently an acid or base, its reactions, particularly with water, lead to the formation of both strong (HCl) and weak (HOCl) acids. Moreover, various chlorine-containing compounds exhibit a spectrum of acid-base properties ranging from strong acids like HCl and perchloric acid to weak bases like sodium hypochlorite. A comprehensive understanding requires considering the specific chemical form of chlorine and the prevailing reaction conditions. This intricate interplay of factors makes chlorine a fascinating element to study within the context of acid-base chemistry. Understanding these nuances is crucial for applications ranging from water purification to industrial processes and environmental considerations.
Latest Posts
Latest Posts
-
How Hot Will Jet Fuel Burn
Mar 10, 2025
-
How Many Dna Combinations Are There
Mar 10, 2025
-
I Am Sorry In French Language
Mar 10, 2025
-
Weight Of A Cubic Foot Of Water
Mar 10, 2025
-
Is A Pencil A Conductor Or Insulator
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about Is Chlorine An Acid Or Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
