Is A Pencil A Conductor Or Insulator
Holbox
Mar 10, 2025 · 5 min read
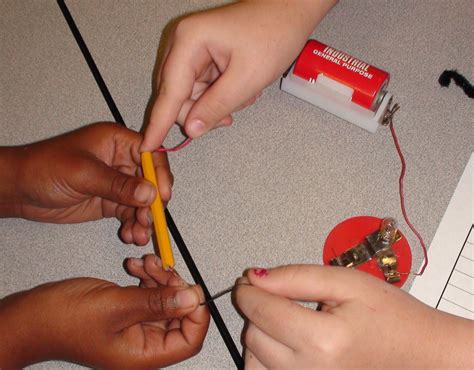
Table of Contents
Is a Pencil a Conductor or an Insulator? Exploring the Electrical Properties of Graphite
The seemingly simple question, "Is a pencil a conductor or an insulator?" opens a fascinating exploration into the world of materials science and electrical conductivity. While the answer isn't a simple yes or no, understanding the nuances requires delving into the composition of pencils, the behavior of electrons, and the factors affecting conductivity. This article will unravel the complexities of a pencil's electrical properties, providing a comprehensive overview for both beginners and those with a deeper interest in physics and materials science.
Understanding Electrical Conductivity
Before examining the pencil, let's establish a clear understanding of electrical conductivity. Electrical conductivity refers to a material's ability to allow the flow of electric current. This flow is facilitated by the movement of electrically charged particles, primarily electrons. Materials are broadly classified into two categories based on their conductivity:
-
Conductors: Materials that readily allow the flow of electric current. They possess a large number of free electrons that can easily move through the material when an electric field is applied. Examples include metals like copper and silver.
-
Insulators: Materials that strongly resist the flow of electric current. They have tightly bound electrons, preventing their easy movement. Examples include rubber, plastic, and wood.
The key difference lies in the electron configuration and bonding structure of the materials. Conductors have delocalized electrons, meaning they are not associated with a particular atom and can move freely within the material's structure. Insulators, conversely, have electrons firmly bound to their respective atoms.
The Composition of a Pencil: Graphite and Clay
A standard pencil isn't made of a single material; it's a mixture. The core, the part we write with, is primarily composed of graphite, a form of carbon. Graphite's structure is layered, with carbon atoms arranged in hexagonal sheets weakly bonded together. This layered structure is crucial to understanding its electrical properties. Mixed with the graphite is clay, which acts as a binder and affects the hardness of the pencil. The ratio of graphite to clay determines the pencil's grade (e.g., 2B, HB, 2H). Softer pencils (higher B grade) contain more graphite, while harder pencils (higher H grade) have more clay.
Graphite: A Unique Material
Graphite's layered structure is key to its electrical properties. While each layer is a strong covalent bond, the interaction between layers is much weaker. This allows for the movement of electrons between layers, contributing to graphite's conductivity. However, it's not as conductive as a metal. The loosely bound electrons in graphite can move more easily than those in an insulator, but not as freely as in metals with a "sea" of delocalized electrons.
Is Graphite a Conductor or an Insulator?
Graphite occupies an interesting middle ground between a conductor and an insulator. It's considered a semiconductor - a material with electrical conductivity intermediate between a conductor and an insulator. This intermediate conductivity is influenced by factors like:
-
Temperature: Conductivity increases with temperature as increased thermal energy allows electrons to overcome energy barriers and move more freely between layers.
-
Pressure: Applying pressure to graphite can increase conductivity by reducing the distance between layers, making electron movement easier.
-
Purity: Impurities within the graphite can reduce conductivity by impeding electron flow.
The Pencil's Electrical Behavior: A Complex Picture
The electrical behavior of a pencil isn't solely determined by the graphite core. The presence of clay, the wood casing, and the surrounding environment all play a role. A pencil, as a whole, is not a highly efficient conductor due to the following factors:
-
Clay: The clay binder in the graphite core acts as an insulator, partially restricting the flow of electrons.
-
Wood: The wooden casing around the graphite core acts as a significant insulator, further limiting electron flow.
-
Surface Contact: The contact between the graphite core and other materials plays a vital role. Imperfect contact can significantly reduce conductivity.
Experimental Demonstrations
To empirically determine a pencil's conductivity, simple experiments can be conducted:
-
Simple Circuit Test: Connecting a pencil lead to a simple circuit with a battery and a light bulb will reveal its limited conductivity. The bulb might glow dimly, if at all, demonstrating that the pencil offers significant resistance to the current flow.
-
Resistance Measurement: Using a multimeter to measure the resistance of a pencil lead will provide a quantitative measurement of its conductivity. The higher the resistance, the lower the conductivity.
Practical Applications: Graphite's Conductivity in Action
Despite its relatively low conductivity compared to metals, graphite's electrical properties are exploited in various applications:
-
Batteries: Graphite is used as an electrode material in many batteries due to its ability to store and release ions.
-
Electrodes: Its conductivity makes it useful in various electrochemical applications.
-
Lubricants: Graphite's layered structure makes it an excellent lubricant, and its conductivity can be beneficial in specific lubricating applications.
Conclusion: Pencil – A Semiconducting Material
In summary, a pencil, primarily composed of graphite and clay, exhibits semiconducting properties. While graphite itself demonstrates some degree of electrical conductivity due to its layered structure and loosely bound electrons, the overall conductivity of the pencil is significantly impacted by the presence of clay, the wooden casing, and the nature of contact points. It's neither a highly efficient conductor nor a complete insulator, but instead, a material with an intermediate level of conductivity, influenced by several factors. Understanding this complex interaction provides a clearer picture of the electrical behavior of this commonplace writing instrument. Further research into the properties of graphite and similar materials will continue to unlock new applications in electronics, energy storage, and other fields. The seemingly simple pencil, therefore, serves as a fascinating example of the complex interplay of materials science and electrical conductivity.
Latest Posts
Latest Posts
-
Cyclemoney Co Latest Posts Categories Finance Money
Mar 10, 2025
-
The Average Variable Cost Per Sale At H
Mar 10, 2025
-
Low Is Too High As Easy Is To
Mar 10, 2025
-
If Is A Linear Transformation Such That And Then
Mar 10, 2025
-
Molar Latent Heat Of The Transformation Caf2
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about Is A Pencil A Conductor Or Insulator . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
