If The Ka Of A Monoprotic Weak Acid Is
Holbox
Mar 20, 2025 · 6 min read
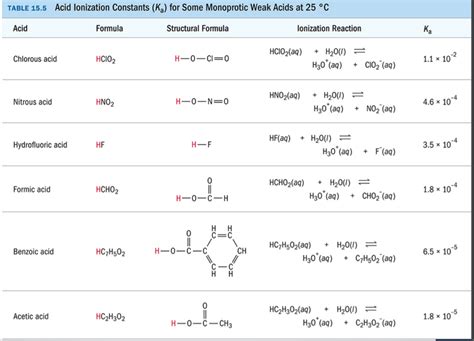
Table of Contents
- If The Ka Of A Monoprotic Weak Acid Is
- Table of Contents
- If the Ka of a Monoprotic Weak Acid Is… Understanding Acid Dissociation and its Implications
- What is Ka and Why is it Important?
- Calculating Ka and pH
- The Relationship Between Ka and pKa
- Applications of Ka and pKa
- 1. Buffer Solutions:
- 2. Pharmaceutical Industry:
- 3. Environmental Science:
- 4. Analytical Chemistry:
- Factors Affecting Ka
- Beyond Monoprotic Acids: Polyprotic Acids
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
If the Ka of a Monoprotic Weak Acid Is… Understanding Acid Dissociation and its Implications
The acid dissociation constant, Ka, is a crucial value in chemistry, particularly when dealing with weak acids. Understanding what Ka represents, how it's calculated, and what it tells us about the behavior of a weak acid in solution is vital for numerous applications, from environmental science to medicine. This comprehensive guide will delve into the significance of Ka for monoprotic weak acids, exploring its implications and applications in detail.
What is Ka and Why is it Important?
The Ka value quantifies the strength of a weak acid. Specifically, it represents the equilibrium constant for the dissociation of a monoprotic weak acid in water. A monoprotic acid is an acid that can donate only one proton (H⁺) per molecule. The general equation for the dissociation of a monoprotic weak acid, HA, is:
HA(aq) ⇌ H⁺(aq) + A⁻(aq)
The equilibrium expression for this reaction, which defines Ka, is:
Ka = [H⁺][A⁻] / [HA]
Where:
- [H⁺] represents the equilibrium concentration of hydrogen ions (in mol/L).
- [A⁻] represents the equilibrium concentration of the conjugate base (in mol/L).
- [HA] represents the equilibrium concentration of the undissociated weak acid (in mol/L).
A larger Ka value indicates a stronger acid, meaning it dissociates more readily in water, producing a higher concentration of H⁺ ions. Conversely, a smaller Ka value indicates a weaker acid, which dissociates less readily and produces a lower concentration of H⁺ ions. The range of Ka values for weak acids is typically much smaller than 1, highlighting their incomplete dissociation.
Calculating Ka and pH
Determining the Ka of a weak acid often involves experimental measurements. One common method involves measuring the pH of a solution of known concentration. Once the pH is known, the hydrogen ion concentration [H⁺] can be calculated using the following equation:
[H⁺] = 10⁻pH
From there, an ICE (Initial, Change, Equilibrium) table can be constructed to determine the equilibrium concentrations of all species involved in the dissociation reaction. These concentrations are then substituted into the Ka expression to calculate the Ka value.
Let's illustrate with an example:
Suppose we have a 0.1 M solution of a weak monoprotic acid, HA, and its pH is measured to be 3.0.
-
Calculate [H⁺]: [H⁺] = 10⁻³⁰ = 1 x 10⁻³ M
-
Construct an ICE table:
| Species | Initial (M) | Change (M) | Equilibrium (M) |
|---|---|---|---|
| HA | 0.1 | -x | 0.1 - x |
| H⁺ | 0 | +x | x |
| A⁻ | 0 | +x | x |
Since pH = 3.0, x = [H⁺] = 1 x 10⁻³ M.
- Calculate Ka:
Ka = [H⁺][A⁻] / [HA] = (1 x 10⁻³)(1 x 10⁻³) / (0.1 - 1 x 10⁻³) ≈ 1 x 10⁻⁵
Therefore, the Ka of this weak acid is approximately 1 x 10⁻⁵.
The Relationship Between Ka and pKa
The pKa is a more convenient way to express the acidity of a weak acid. It's defined as the negative logarithm (base 10) of the Ka value:
pKa = -log₁₀(Ka)
The pKa scale is easier to interpret than the Ka scale. A lower pKa value indicates a stronger acid, while a higher pKa value indicates a weaker acid. This is the opposite trend compared to the Ka scale. For example, an acid with a pKa of 2 is stronger than an acid with a pKa of 5.
Applications of Ka and pKa
Understanding Ka and pKa is crucial in various fields:
1. Buffer Solutions:
Buffer solutions resist changes in pH upon the addition of small amounts of acid or base. They are typically composed of a weak acid and its conjugate base (or a weak base and its conjugate acid). The optimal buffering capacity occurs when the pH of the buffer is close to the pKa of the weak acid. This is because the ratio of [A⁻]/[HA] is approximately 1 at the pKa, providing maximum resistance to pH change. The Henderson-Hasselbalch equation is often used to calculate the pH of a buffer solution:
pH = pKa + log₁₀([A⁻]/[HA])
2. Pharmaceutical Industry:
Many drugs are weak acids or bases. Knowing their Ka or pKa values is critical for determining their solubility, absorption, and distribution in the body. The pH of the environment significantly impacts drug bioavailability.
3. Environmental Science:
The acidity of natural water bodies (e.g., lakes, rivers) is crucial for aquatic life. The Ka values of weak acids present in the environment, such as carbonic acid (H₂CO₃), help determine the pH and overall chemical composition of these systems. Acid rain, for instance, is a significant environmental issue related to the dissociation of weak acids in the atmosphere.
4. Analytical Chemistry:
Ka values are essential in various analytical techniques, such as titrations. The equivalence point in the titration of a weak acid with a strong base can be determined using the Ka value and the concentration of the acid.
Factors Affecting Ka
Several factors can influence the Ka value of a monoprotic weak acid:
-
Structure of the acid: The presence of electron-withdrawing groups increases the acidity and, consequently, the Ka value. Electron-donating groups have the opposite effect. The strength of the acid is also related to the stability of the conjugate base. A more stable conjugate base leads to a stronger acid and a higher Ka.
-
Temperature: Ka typically increases with increasing temperature, signifying a greater degree of dissociation at higher temperatures. This is because the dissociation of weak acids is usually an endothermic process.
-
Solvent: The solvent in which the acid is dissolved can significantly impact its dissociation. Polar solvents tend to enhance dissociation, leading to higher Ka values.
Beyond Monoprotic Acids: Polyprotic Acids
While this discussion focuses on monoprotic weak acids, many acids can donate more than one proton. These are called polyprotic acids. Polyprotic acids have multiple Ka values, one for each dissociation step. For example, carbonic acid (H₂CO₃) has two Ka values, one for the loss of the first proton and another for the loss of the second proton. The different Ka values reflect the varying ease of losing each successive proton. The first proton is typically easier to lose than subsequent protons.
Conclusion
The acid dissociation constant, Ka, is a fundamental concept in chemistry that provides critical insights into the behavior of weak acids in solution. Understanding how to calculate Ka, its relationship to pKa, and its applications in various fields, from pharmaceuticals to environmental science, is essential for anyone studying chemistry or related disciplines. The ability to interpret Ka values allows for accurate predictions of pH, equilibrium concentrations, and the overall behavior of weak acids in different contexts. Further investigation into the factors influencing Ka, including structural effects, temperature, and solvent, can provide even greater control and understanding of acid-base reactions. Finally, extending this knowledge to polyprotic acids will provide a more comprehensive understanding of acid-base chemistry in complex systems.
Latest Posts
Latest Posts
-
Unlike Mediation Conciliation Attempts To Do Which Of The Following
Mar 27, 2025
-
Which Of The Following Does Not Belong To Holding Costs
Mar 27, 2025
-
Consider The Three Electromagnetic Waves Shown In The Image
Mar 27, 2025
-
Family Furniture Corporation Incurred The Following Costs
Mar 27, 2025
-
Which Describes The Cost To Produce One Item
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about If The Ka Of A Monoprotic Weak Acid Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
