How Many Valence Electrons Does Gold Have
Holbox
Mar 15, 2025 · 6 min read
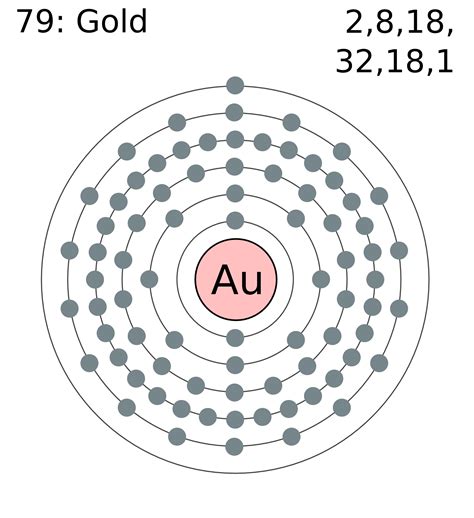
Table of Contents
How Many Valence Electrons Does Gold Have? Unraveling the Chemistry of a Precious Metal
Gold, a lustrous, malleable, and ductile metal known for its beauty and resistance to corrosion, has captivated humanity for millennia. Its unique properties, however, stem from its underlying electronic structure, particularly the number of valence electrons it possesses. This article delves deep into the electronic configuration of gold, explaining its valence electron count and how it influences its chemical behavior and diverse applications.
Understanding Valence Electrons: The Key to Chemical Reactivity
Before we explore gold's valence electrons, let's establish a fundamental understanding of what valence electrons are. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are crucial because they are the ones most readily involved in chemical bonding. The number of valence electrons an atom possesses dictates its reactivity, the types of bonds it can form (ionic, covalent, metallic), and its overall chemical behavior. Atoms strive to achieve a stable electron configuration, often by gaining, losing, or sharing valence electrons to attain a full outer shell (usually eight electrons, following the octet rule, though exceptions exist).
Determining Gold's Electronic Configuration
To determine the number of valence electrons in gold (Au), we need to examine its electronic configuration. Gold's atomic number is 79, meaning it has 79 protons and 79 electrons in a neutral atom. The electronic configuration is determined by filling the electron orbitals according to the Aufbau principle and Hund's rule. This results in the following electronic configuration for gold:
[Xe] 4f<sup>14</sup> 5d<sup>10</sup> 6s<sup>1</sup>
This configuration reveals the distribution of electrons across different energy levels and sublevels. The [Xe] represents the core electrons, which are the electrons filling the orbitals up to Xenon's electron configuration. These core electrons are relatively stable and do not significantly participate in chemical bonding. The electrons that truly matter for chemical reactivity are the ones in the outermost shell, which in gold's case are found in the 6s and 5d orbitals.
Gold's Valence Electrons: The Relativistic Effect
While one might initially assume gold has only one valence electron (the 6s<sup>1</sup> electron), the situation is more complex due to the relativistic effect. Relativistic effects become significant for heavy atoms like gold. The inner electrons move at such high speeds (a significant fraction of the speed of light) that their mass increases according to Einstein's theory of special relativity. This increase in mass causes the inner electrons' orbitals to contract, thereby shielding the outer electrons less effectively.
This reduced shielding has a profound impact on the 6s and 5d orbitals. The 6s orbital is contracted more significantly than the 5d orbitals. This makes the 6s electron less easily removed than might be predicted based on simple atomic theory. However, the 5d electrons are also more readily available for bonding compared to other elements with similar electron configurations due to the relativistic effect, essentially lowering their energy level and making them more accessible for interaction.
The Role of Relativistic Effects in Gold's Properties
The relativistic contraction of the 6s orbital explains many of gold's unique properties:
-
Color: Unlike most other metals, gold exhibits a distinctive yellow color. This is attributed to the relativistic effects which alter the energy gap between the d and s orbitals. The resulting electronic transitions absorb blue light, reflecting yellow light.
-
Chemical Inertness: While gold does react with certain substances under specific conditions (like aqua regia), it is generally considered chemically inert. The relativistic effects help stabilize the 6s electron making it less prone to oxidation compared to other transition metals.
-
High Density: The relativistic contraction of the orbitals leads to a smaller atomic radius, resulting in a higher density for gold compared to other metals in its group.
-
Malleability and Ductility: Gold's metallic bonding is influenced by the interaction of the 6s and 5d electrons. Relativistic effects fine-tune these interactions, contributing to gold's exceptional malleability and ductility.
So, How Many Valence Electrons Does Gold Have?
The answer to the question “how many valence electrons does gold have?” is nuanced. While strictly speaking, the 6s<sup>1</sup> electron is the outermost electron, the influence of the 5d<sup>10</sup> electrons cannot be ignored due to relativistic effects. These 5d electrons are effectively valence electrons as well, participating in chemical bonding. Therefore, it is most accurate to consider gold as having one to eleven valence electrons. The exact number depends on the specific chemical environment and the type of bonding involved.
Gold's Chemical Behavior: A Consequence of its Valence Electrons
Gold's chemical behavior is a direct consequence of its valence electron configuration and the relativistic effects. It predominantly exhibits a +1 or +3 oxidation state in its compounds. The +1 oxidation state involves the loss of the 6s<sup>1</sup> electron. The +3 oxidation state is more complex and involves the participation of 5d electrons, often showcasing relativistic effects that fine-tune its properties.
Gold's Applications: Leveraging its Unique Properties
Gold's unique combination of chemical inertness, malleability, ductility, and conductivity underpins its wide range of applications. These applications include:
-
Jewelry and Decorative Arts: Gold's beauty and resistance to corrosion make it highly valued for jewelry and decorative purposes.
-
Electronics: Gold's excellent conductivity and resistance to corrosion are exploited in electronics for contacts, connectors, and integrated circuits.
-
Medicine: Gold compounds are employed in certain medications for treating rheumatoid arthritis and other inflammatory diseases.
-
Catalysis: Gold nanoparticles exhibit remarkable catalytic activity in various chemical reactions, owing to their unique electronic and structural properties influenced by the relativistic effects.
-
Investment: Gold's rarity and stability make it a favored investment asset.
Conclusion: Beyond a Simple Number
Determining the exact number of valence electrons for gold isn't a straightforward case of simply counting electrons in the outermost shell. The relativistic effects significantly impact the energy levels and participation of both 6s and 5d electrons in chemical bonding. Considering the significant influence of relativistic effects, it’s most appropriate to conclude that gold has a variable number of valence electrons, ranging from one to eleven, making it a unique and fascinating element with diverse applications stemming from its complex electronic structure. Understanding these nuances is key to appreciating gold's exceptional properties and its widespread use across various fields. Further research continues to explore the intricate interplay of relativistic effects and valence electron behavior in gold, leading to new discoveries and applications of this precious metal.
Latest Posts
Latest Posts
-
Standing Waves On A String Lab Report Chegg
Mar 15, 2025
-
How Do You Ask A Question On Chegg
Mar 15, 2025
-
Can You Highlight In A Chegg Rental
Mar 15, 2025
-
Which Is The Base Peak Chegg
Mar 15, 2025
-
How Long Does Chegg Take To Ship
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Gold Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
