How Many Valence Electrons Does Ca Have
Holbox
Mar 20, 2025 · 5 min read
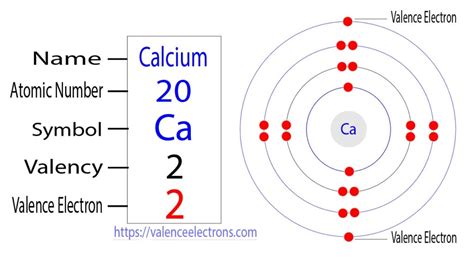
Table of Contents
How Many Valence Electrons Does Calcium (Ca) Have? A Deep Dive into Atomic Structure and Chemical Bonding
Understanding valence electrons is crucial for grasping the fundamentals of chemistry. These outermost electrons determine an atom's reactivity and how it interacts with other atoms to form molecules and compounds. This article delves into the question: how many valence electrons does calcium (Ca) have? We'll explore the atomic structure of calcium, its electron configuration, and how this configuration dictates its chemical behavior. Furthermore, we'll examine the implications of calcium's valence electrons in various chemical contexts.
Understanding Valence Electrons
Before we pinpoint the number of valence electrons in calcium, let's establish a clear understanding of what valence electrons are. Valence electrons are the electrons located in the outermost electron shell (also known as the valence shell) of an atom. These electrons are the ones most readily involved in chemical bonding. They are the primary participants in the formation of chemical bonds, which hold atoms together in molecules and compounds. The number of valence electrons dictates an atom's chemical properties and reactivity.
Atoms strive for stability, often achieved by having a full outermost electron shell. This usually means eight electrons (the octet rule), although there are exceptions, particularly with elements in the first and second rows of the periodic table. Atoms will either gain, lose, or share electrons to achieve this stable configuration. This process determines the type of chemical bonds formed – ionic, covalent, or metallic.
Calcium's Position on the Periodic Table
Calcium (Ca) is an element located in Group 2 (also known as the alkaline earth metals) and Period 4 of the periodic table. Its atomic number is 20, meaning it has 20 protons and, in a neutral atom, 20 electrons. The periodic table's organization is not arbitrary; it reflects the underlying atomic structure and electron configuration of the elements. The group number (for the representative elements) generally indicates the number of valence electrons.
Calcium's Electron Configuration and Valence Electrons
To determine the number of valence electrons in calcium, we need to examine its electron configuration. The electron configuration describes how electrons are distributed among the various energy levels (shells and subshells) within an atom. Calcium's electron configuration is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s²
Let's break this down:
- 1s²: Two electrons in the first energy level (shell), the innermost shell.
- 2s² 2p⁶: Eight electrons in the second energy level. The 's' subshell holds two electrons, and the 'p' subshell holds six.
- 3s² 3p⁶: Eight electrons in the third energy level.
- 4s²: Two electrons in the fourth energy level.
The outermost shell for calcium is the fourth energy level (n=4), which contains two electrons in the 4s subshell. Therefore, calcium has 2 valence electrons.
Visualizing Calcium's Electron Configuration
Imagine the electrons arranged in concentric circles around the nucleus, representing the energy levels. The innermost circle holds two electrons (1s²), the next circle holds eight (2s² 2p⁶), the following circle holds eight (3s² 3p⁶), and the outermost circle holds the two valence electrons (4s²).
Calcium's Chemical Behavior and Valence Electrons
Calcium's two valence electrons are key to understanding its chemical behavior. Because it's easier for calcium to lose two electrons than to gain six to achieve a stable octet, it tends to lose these two electrons to form a +2 ion (Ca²⁺). This process is called ionization. The loss of these electrons leaves calcium with a stable electron configuration matching the noble gas Argon (Ar).
This tendency to lose electrons makes calcium highly reactive, particularly with nonmetals and water. It readily forms ionic bonds with electronegative elements such as chlorine (Cl), oxygen (O), and sulfur (S). These bonds arise from the electrostatic attraction between the positively charged calcium ion (Ca²⁺) and the negatively charged ions of nonmetals.
Examples of Calcium's Chemical Reactions
- Reaction with Chlorine: Calcium reacts vigorously with chlorine to form calcium chloride (CaCl₂). Each calcium atom loses two electrons to two chlorine atoms, each of which gains one electron to form chloride ions (Cl⁻).
- Reaction with Oxygen: Calcium reacts with oxygen to form calcium oxide (CaO). Again, calcium loses two electrons, and oxygen gains two electrons to form oxide ions (O²⁻).
- Reaction with Water: Calcium reacts slowly with water to produce calcium hydroxide (Ca(OH)₂) and hydrogen gas (H₂). This reaction demonstrates calcium's reactivity with water, releasing hydrogen gas as a byproduct.
Applications of Calcium and its Compounds
Calcium's chemical properties, dictated by its two valence electrons, lead to numerous applications in various fields:
- Biological Systems: Calcium plays a vital role in biological systems. It's essential for bone and teeth formation, muscle contraction, nerve impulse transmission, and blood clotting.
- Construction Materials: Calcium compounds such as calcium carbonate (CaCO₃, limestone) are used extensively in construction as building materials like cement and concrete.
- Metallurgy: Calcium is used as a reducing agent in the extraction of some metals from their ores.
- Industrial Applications: Calcium compounds find use in various industrial processes, including water treatment, paper manufacturing, and food processing.
Conclusion: The Significance of Calcium's Two Valence Electrons
In conclusion, calcium (Ca) possesses two valence electrons, located in its outermost electron shell (4s²). These two electrons determine calcium's chemical reactivity and its ability to form ionic bonds by losing these electrons to achieve a stable electron configuration. This fundamental characteristic explains its behavior in chemical reactions and its significance in biological and industrial applications. Understanding valence electrons is essential to comprehend the chemical properties and behavior of all elements, and calcium serves as an excellent example of this principle in action. The simplicity of its electron configuration, coupled with its widespread importance, makes it an ideal case study in the world of chemistry. Further investigation into other elements and their valence electron configurations will only enhance the understanding of chemical bonding and reactivity.
Latest Posts
Latest Posts
-
What Is Meant By The Phrase Spreading The Overhead
Mar 21, 2025
-
The Hydrolysis Of Esters Amides And Nitriles
Mar 21, 2025
-
What Is One Of The Rules Of A Measure
Mar 21, 2025
-
Why Are Collagen Fibers A Critical Component Of Bone
Mar 21, 2025
-
An Ionic Bond Is Best Described As
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Ca Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
