An Ionic Bond Is Best Described As
Holbox
Mar 21, 2025 · 6 min read
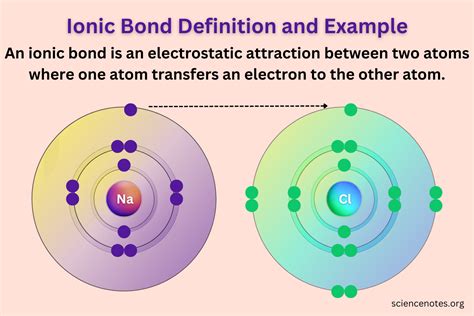
Table of Contents
- An Ionic Bond Is Best Described As
- Table of Contents
- An Ionic Bond is Best Described as: A Deep Dive into Electrostatic Attraction
- The Genesis of an Ionic Bond: Electron Transfer and Octet Rule
- Electronegativity: The Driving Force
- Ion Formation: Cations and Anions
- The Electrostatic Attraction: The Heart of the Ionic Bond
- Crystal Lattice: An Ordered Arrangement
- Ionic Compounds: Properties and Characteristics
- Beyond the Basics: Factors Influencing Ionic Bond Strength
- Examples of Ionic Compounds and Their Applications
- Ionic Bonds vs. Other Bond Types: Covalent and Metallic
- Conclusion: The Significance of Ionic Bonds
- Latest Posts
- Latest Posts
- Related Post
An Ionic Bond is Best Described as: A Deep Dive into Electrostatic Attraction
An ionic bond, at its simplest, is the electrostatic attraction between two oppositely charged ions. This seemingly straightforward definition belies the complexity and fascinating nuances of this fundamental chemical interaction, crucial for understanding the behavior of a vast array of materials, from table salt to sophisticated electronics. This comprehensive exploration will delve into the intricacies of ionic bonds, examining their formation, properties, and significance in various fields.
The Genesis of an Ionic Bond: Electron Transfer and Octet Rule
The formation of an ionic bond hinges on the transfer of electrons between atoms. This transfer doesn't occur randomly; it's driven by the fundamental desire of atoms to achieve a stable electron configuration, typically following the octet rule. The octet rule dictates that atoms tend to gain, lose, or share electrons to acquire a full outer electron shell of eight electrons, mimicking the stable electron configuration of noble gases.
Electronegativity: The Driving Force
The concept of electronegativity plays a pivotal role in determining whether an ionic bond will form. Electronegativity is a measure of an atom's ability to attract electrons towards itself within a chemical bond. A significant difference in electronegativity between two atoms is essential for ionic bond formation.
When a highly electronegative atom (like a halogen such as chlorine) encounters an atom with low electronegativity (like an alkali metal such as sodium), the electronegative atom exerts a strong pull on the valence electrons of the less electronegative atom. This pull is so strong that the valence electron(s) are completely transferred from the less electronegative atom to the more electronegative atom.
Ion Formation: Cations and Anions
This electron transfer results in the formation of ions:
-
Cations: The atom that loses electrons becomes positively charged because it now has more protons than electrons. These positively charged ions are called cations. For example, sodium (Na) loses one electron to become a sodium cation (Na⁺).
-
Anions: The atom that gains electrons becomes negatively charged because it now has more electrons than protons. These negatively charged ions are called anions. For example, chlorine (Cl) gains one electron to become a chloride anion (Cl⁻).
The Electrostatic Attraction: The Heart of the Ionic Bond
Once the cations and anions are formed, the opposite charges attract each other through a powerful electrostatic force. This electrostatic attraction is the essence of the ionic bond, holding the ions together in a stable crystal lattice structure.
Crystal Lattice: An Ordered Arrangement
The ions don't just randomly clump together; they arrange themselves in a highly ordered three-dimensional structure called a crystal lattice. This arrangement is optimized to minimize repulsive forces between like charges (e.g., cation-cation, anion-anion) and maximize attractive forces between opposite charges (cation-anion). The specific arrangement of ions in the crystal lattice depends on the size and charge of the ions involved.
Ionic Compounds: Properties and Characteristics
The properties of ionic compounds are directly related to their ionic bonding and crystal lattice structure. Several key characteristics distinguish ionic compounds:
-
High Melting and Boiling Points: The strong electrostatic forces between ions require a significant amount of energy to overcome, resulting in high melting and boiling points.
-
Hardness and Brittleness: While many ionic compounds are relatively hard, they are also brittle. When subjected to stress, the aligned layers of ions can shift, causing like charges to align and resulting in a repulsive force that causes the crystal to fracture.
-
Solubility in Polar Solvents: Ionic compounds are often soluble in polar solvents like water. The polar solvent molecules can surround and interact with the ions, weakening the electrostatic attraction and allowing the ions to dissolve.
-
Electrical Conductivity: Ionic compounds are generally poor conductors of electricity in their solid state because the ions are fixed in the crystal lattice. However, when molten (melted) or dissolved in water, they become good conductors because the ions are free to move and carry an electric current.
Beyond the Basics: Factors Influencing Ionic Bond Strength
While the fundamental principle of electrostatic attraction governs ionic bonding, several factors influence the strength of the bond:
-
Charge of the Ions: Higher charges on the ions lead to stronger electrostatic attractions and therefore stronger ionic bonds. For instance, the bond in magnesium oxide (MgO) where Mg²⁺ and O²⁻ ions are involved is stronger than the bond in sodium chloride (NaCl), where the ions have a +1 and -1 charge, respectively.
-
Size of the Ions: Smaller ions result in stronger ionic bonds because the electrostatic force is inversely proportional to the square of the distance between the ions. Smaller ions are closer together, leading to stronger attraction.
-
Lattice Energy: Lattice energy is a measure of the strength of the ionic bond and the stability of the crystal lattice. It represents the energy released when gaseous ions combine to form a solid ionic compound. Higher lattice energy signifies a stronger ionic bond.
Examples of Ionic Compounds and Their Applications
Ionic compounds are ubiquitous in nature and have a wide range of applications. Here are a few examples:
-
Sodium Chloride (NaCl): Common table salt, crucial for human health and widely used in food preservation and industrial processes.
-
Calcium Carbonate (CaCO₃): A major component of limestone, marble, and chalk, used in construction materials, cement production, and as an antacid.
-
Magnesium Oxide (MgO): Used as a refractory material, in insulation, and in various chemical applications.
-
Potassium Chloride (KCl): Used as a fertilizer, in medicine (electrolyte replacement), and in various industrial applications.
-
Silver Chloride (AgCl): Used in photography and as an antiseptic.
Ionic Bonds vs. Other Bond Types: Covalent and Metallic
It's important to differentiate ionic bonds from other types of chemical bonds:
-
Covalent Bonds: In covalent bonds, atoms share electrons rather than transferring them. This type of bonding occurs between atoms with similar electronegativities. Covalent bonds typically form between nonmetals.
-
Metallic Bonds: Metallic bonds involve the sharing of delocalized electrons among a lattice of metal atoms. This creates a "sea" of electrons that allows for high electrical and thermal conductivity.
Conclusion: The Significance of Ionic Bonds
Ionic bonds represent a fundamental and prevalent type of chemical interaction with far-reaching consequences. Understanding their formation, properties, and the factors influencing their strength is paramount in various scientific disciplines, including chemistry, materials science, and geology. From the salt on our tables to the intricate structures of minerals and the functionality of numerous materials, ionic bonds play a critical role in shaping our world. The seemingly simple electrostatic attraction between ions unveils a fascinating world of complex interactions and remarkable properties, underscoring the beauty and elegance of fundamental chemical principles. Further exploration into the diverse applications and ongoing research related to ionic compounds will undoubtedly reveal even more about the importance of this essential chemical bond.
Latest Posts
Latest Posts
-
All Of The Following Are Features Of Managerial Accounting Except
Mar 28, 2025
-
Garbage Containers Used By Operation Should Be
Mar 28, 2025
-
Minimum Requirement For Cleaning Juice Bar Blender
Mar 28, 2025
-
The Graph Shows The X Directed Force
Mar 28, 2025
-
Use The Graph To Answer The Following Questions
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about An Ionic Bond Is Best Described As . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
