How Do Fats Differ From Proteins Nucleic Acids And Polysaccharides
Holbox
Mar 21, 2025 · 6 min read
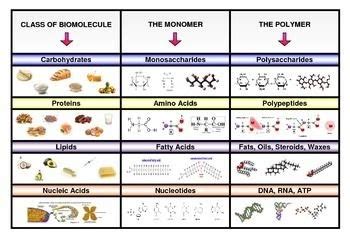
Table of Contents
- How Do Fats Differ From Proteins Nucleic Acids And Polysaccharides
- Table of Contents
- How Do Fats Differ From Proteins, Nucleic Acids, and Polysaccharides?
- 1. Fats (Lipids) vs. Proteins: A Tale of Two Structures
- 1.1. Building Blocks:
- 1.2. Structure and Function:
- 1.3. Key Differences Summarized:
- 2. Fats (Lipids) vs. Nucleic Acids: Information Storage vs. Energy Storage
- 2.1. Building Blocks:
- 2.2. Structure and Function:
- 2.3. Key Differences Summarized:
- 3. Fats (Lipids) vs. Polysaccharides: Energy Storage and Structural Support
- 3.1. Building Blocks:
- 3.2. Structure and Function:
- 3.3. Key Differences Summarized:
- 4. Comparing all Four Macromolecule Classes: A Comprehensive Overview
- 5. Conclusion: The Interdependence of Macromolecules
- Latest Posts
- Latest Posts
- Related Post
How Do Fats Differ From Proteins, Nucleic Acids, and Polysaccharides?
Biological macromolecules are the large complex molecules essential for life. They're categorized into four major classes: carbohydrates (including polysaccharides), lipids (fats), proteins, and nucleic acids. While all are crucial for cellular function, they differ dramatically in their structure, function, and properties. This article delves deep into the distinctions between fats (lipids) and the other three macromolecule classes, highlighting their unique characteristics and biological roles.
1. Fats (Lipids) vs. Proteins: A Tale of Two Structures
The fundamental difference between fats and proteins lies in their building blocks and the resulting structures.
1.1. Building Blocks:
-
Fats (Lipids): Primarily composed of glycerol and fatty acids. Glycerol is a three-carbon alcohol, while fatty acids are long hydrocarbon chains with a carboxyl group (-COOH) at one end. The fatty acids can be saturated (no double bonds between carbons), monounsaturated (one double bond), or polyunsaturated (multiple double bonds). The arrangement and type of fatty acids significantly influence the lipid's properties.
-
Proteins: Constructed from amino acids. Amino acids are characterized by a central carbon atom bonded to an amino group (-NH2), a carboxyl group (-COOH), a hydrogen atom, and a unique side chain (R group). The sequence and arrangement of these amino acids determine the protein's unique three-dimensional structure and function.
1.2. Structure and Function:
-
Fats (Lipids): Lipids are generally nonpolar and hydrophobic (water-repelling). They exist in various forms, including triglycerides (the most common type, consisting of glycerol bonded to three fatty acids), phospholipids (major components of cell membranes), and steroids (like cholesterol). Their functions include energy storage, insulation, protection of organs, and forming cell membranes.
-
Proteins: Proteins exhibit diverse structures, ranging from simple linear chains (primary structure) to complex three-dimensional shapes (secondary, tertiary, and quaternary structures). These complex structures enable them to perform a vast array of functions, including catalysis (enzymes), transport (hemoglobin), structural support (collagen), movement (actin and myosin), defense (antibodies), and cell signaling.
1.3. Key Differences Summarized:
| Feature | Fats (Lipids) | Proteins |
|---|---|---|
| Monomers | Glycerol and fatty acids | Amino acids |
| Polarity | Nonpolar, hydrophobic | Polarity varies depending on amino acid side chains |
| Solubility | Insoluble in water | Solubility varies depending on amino acid side chains |
| Primary Function | Energy storage, insulation, membrane structure | Diverse functions: catalysis, transport, structure, etc. |
| Structure | Relatively simple structures (triglycerides) | Complex 3D structures |
2. Fats (Lipids) vs. Nucleic Acids: Information Storage vs. Energy Storage
The contrast between fats and nucleic acids is stark, reflecting their fundamentally different roles within the cell.
2.1. Building Blocks:
-
Fats (Lipids): As previously discussed, lipids are built from glycerol and fatty acids.
-
Nucleic Acids: Composed of nucleotides. Each nucleotide consists of a sugar (ribose in RNA or deoxyribose in DNA), a phosphate group, and a nitrogenous base (adenine, guanine, cytosine, thymine (in DNA), or uracil (in RNA)).
2.2. Structure and Function:
-
Fats (Lipids): Lipids are primarily involved in energy storage and structural components of membranes. Their relatively simple structure reflects their less complex functions.
-
Nucleic Acids: Nucleic acids, DNA and RNA, are responsible for storing and transmitting genetic information. DNA's double helix structure allows for the efficient storage of vast amounts of genetic code. RNA plays diverse roles in gene expression, including carrying genetic information (mRNA), forming ribosomes (rRNA), and facilitating protein synthesis (tRNA).
2.3. Key Differences Summarized:
| Feature | Fats (Lipids) | Nucleic Acids (DNA & RNA) |
|---|---|---|
| Monomers | Glycerol and fatty acids | Nucleotides |
| Primary Function | Energy storage, membrane structure | Information storage and transmission |
| Structure | Relatively simple | Complex (DNA double helix, RNA various forms) |
| Information Content | None | High (genetic code) |
3. Fats (Lipids) vs. Polysaccharides: Energy Storage and Structural Support
Both fats and polysaccharides serve as energy storage molecules, but their properties and efficiency differ considerably.
3.1. Building Blocks:
-
Fats (Lipids): Glycerol and fatty acids.
-
Polysaccharides: Composed of monosaccharides (simple sugars) linked together. Common examples include starch (glucose polymer in plants), glycogen (glucose polymer in animals), and cellulose (glucose polymer in plant cell walls).
3.2. Structure and Function:
-
Fats (Lipids): Efficient energy storage due to their high energy density. They are hydrophobic, making them excellent for compact energy storage without attracting water molecules that add weight.
-
Polysaccharides: Also store energy, but less efficiently than fats. Starch and glycogen are readily broken down to release glucose for energy. Cellulose, however, serves a primarily structural role, providing rigidity to plant cell walls.
3.3. Key Differences Summarized:
| Feature | Fats (Lipids) | Polysaccharides (e.g., starch, glycogen, cellulose) |
|---|---|---|
| Monomers | Glycerol and fatty acids | Monosaccharides (simple sugars) |
| Primary Function | Energy storage, membrane structure | Energy storage (starch, glycogen), structural support (cellulose) |
| Energy Density | High | Lower than fats |
| Solubility | Insoluble in water | Solubility varies depending on the polysaccharide |
| Structure | Relatively simple | Complex, branched or linear structures |
4. Comparing all Four Macromolecule Classes: A Comprehensive Overview
Let's summarize the key distinctions between fats, proteins, nucleic acids, and polysaccharides in a comprehensive table:
| Feature | Fats (Lipids) | Proteins | Nucleic Acids (DNA & RNA) | Polysaccharides (e.g., starch, glycogen, cellulose) |
|---|---|---|---|---|
| Monomers | Glycerol and fatty acids | Amino acids | Nucleotides | Monosaccharides (simple sugars) |
| Primary Function | Energy storage, insulation, membrane structure | Diverse functions: catalysis, transport, structure, etc. | Information storage and transmission | Energy storage (starch, glycogen), structural support (cellulose) |
| Structure | Relatively simple | Complex 3D structures | Complex (DNA double helix, RNA various forms) | Complex, branched or linear structures |
| Polarity | Nonpolar, hydrophobic | Polarity varies depending on amino acid side chains | Polar, hydrophilic | Polarity varies depending on the polysaccharide |
| Solubility | Insoluble in water | Solubility varies depending on amino acid side chains | Soluble in water | Solubility varies depending on the polysaccharide |
| Energy Storage | High energy density | No significant energy storage | No significant energy storage | Moderate energy density |
| Information Storage | None | No significant information storage | High (genetic code) | None |
5. Conclusion: The Interdependence of Macromolecules
While these four classes of macromolecules have distinct properties and functions, they are intricately interconnected within a living organism. Proteins are synthesized based on the genetic information encoded in nucleic acids. Both proteins and lipids are essential components of cell membranes. Polysaccharides can be broken down to provide energy that fuels metabolic processes, many of which are catalyzed by proteins. This interconnectedness highlights the remarkable complexity and elegance of biological systems. Understanding the unique characteristics of each macromolecule class is crucial for comprehending the fundamental processes of life.
Latest Posts
Latest Posts
-
Tech Solutions Is A Consulting Firm
Mar 28, 2025
-
Based On The Curves What Tenor Should A Ranked
Mar 28, 2025
-
Which Of The Following Is Not An Advantage Of Budgeting
Mar 28, 2025
-
Evaluate The Integral By Changing To Cylindrical Coordinates
Mar 28, 2025
-
Which Statement Correctly Describes The Origin Of Lymph Fluid
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How Do Fats Differ From Proteins Nucleic Acids And Polysaccharides . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
