Given The Planar Trisubstituted Cyclohexane Below
Holbox
Mar 19, 2025 · 5 min read
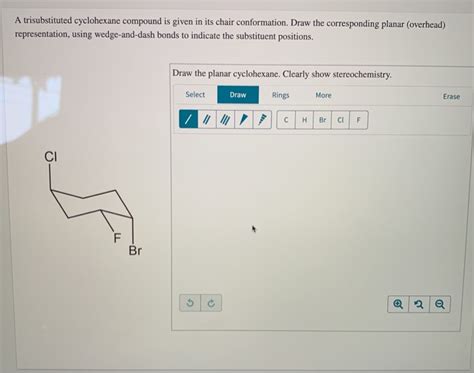
Table of Contents
Conformational Analysis of Trisubstituted Cyclohexanes: A Deep Dive into Planarity and Stability
The conformational analysis of cyclohexane derivatives is a cornerstone of organic chemistry, impacting diverse fields from drug design to materials science. Understanding the factors governing conformational preferences is crucial for predicting reactivity and physical properties. This article focuses on the complexities introduced by trisubstituted cyclohexanes, specifically examining scenarios where apparent planarity might be observed, and the implications for stability and reactivity.
Understanding Cyclohexane Conformations
Before delving into trisubstituted systems, let's revisit the fundamental conformations of cyclohexane: the chair and boat conformations. The chair conformation is significantly more stable due to the absence of flagpole interactions and 1,3-diaxial interactions present in the boat conformation. The chair conformation possesses two types of hydrogen atoms: axial and equatorial. Axial hydrogens are oriented perpendicular to the plane of the ring, while equatorial hydrogens lie approximately in the plane.
Substituent Effects on Conformational Preference
Introducing substituents to the cyclohexane ring introduces steric interactions that influence conformational equilibria. Bulky substituents strongly prefer the equatorial position to minimize 1,3-diaxial interactions. This preference is quantified by the A-value, representing the difference in free energy between axial and equatorial conformations. Larger A-values indicate a stronger preference for the equatorial position.
The Challenge of Planar Trisubstituted Cyclohexanes
The concept of a "planar" trisubstituted cyclohexane is inherently paradoxical. Cyclohexane's inherent flexibility dictates that a perfectly planar conformation is extremely high in energy, if not impossible, to achieve. However, specific substituent combinations and intramolecular interactions can lead to conformations that appear closer to planarity than the standard chair form.
Factors Influencing Apparent Planarity
Several factors contribute to a deviation from the typical chair conformation in trisubstituted cyclohexanes, leading to what might be perceived as planarity:
-
Strong Intramolecular Interactions: The presence of strong interactions, such as hydrogen bonds or other attractive forces between substituents, can override the inherent preference for a chair conformation. These interactions might force the ring into a less stable, but more conformationally restricted, geometry.
-
Steric Constraints: Bulky substituents in specific positions can create significant steric clashes in the chair conformation. To alleviate this strain, the ring might adopt a distorted conformation that appears partially flattened. This is particularly likely if the substituents are positioned 1,2, or 1,3 to each other.
-
Ring Fusion in Polycyclic Systems: When the cyclohexane ring is part of a larger polycyclic system, the constraints imposed by the fused rings might significantly restrict the conformational freedom of the cyclohexane ring, leading to a distorted conformation.
Analyzing Specific Examples: Hypothetical Trisubstituted Cyclohexanes
Let's consider several hypothetical examples to illustrate these concepts. Note that without a specific structure provided, we'll explore general scenarios.
Example 1: 1,2,3-Trisubstituted Cyclohexane with Bulky Substituents
Imagine a 1,2,3-trisubstituted cyclohexane with three large, bulky substituents. The chair conformation would experience significant 1,3-diaxial interactions, particularly if all three substituents attempted to adopt equatorial positions. To mitigate this steric strain, the ring might adopt a twist-boat conformation or a significantly distorted chair conformation, appearing partially flattened. The precise conformation would depend on the size and nature of the substituents and would require computational modeling for accurate prediction.
Example 2: 1,3,5-Trisubstituted Cyclohexane with Hydrogen Bonding Potential
Consider a 1,3,5-trisubstituted cyclohexane where two of the substituents can engage in hydrogen bonding. For instance, one substituent could be a hydroxyl group (-OH) and another a carbonyl group (C=O). The potential for hydrogen bond formation between these substituents could significantly influence the preferred conformation. The ring might adopt a conformation that brings the hydrogen bond donors and acceptors into close proximity, possibly leading to a flattened conformation to optimize the hydrogen bond interaction.
Example 3: Trisubstituted Cyclohexane within a Fused Ring System
When a trisubstituted cyclohexane is part of a fused bicyclic or polycyclic system, the conformational possibilities become severely limited. The fusion itself dictates the overall shape and conformation. The trisubstituted cyclohexane ring might be forced into a significantly distorted, partially planar conformation to accommodate the fusion. The degree of planarity depends on the size and structure of the fused ring system.
Determining Conformational Preferences: Experimental and Computational Methods
Determining the actual conformation of a trisubstituted cyclohexane requires a combination of experimental and computational techniques:
-
Nuclear Magnetic Resonance (NMR) Spectroscopy: NMR spectroscopy, particularly 1H and 13C NMR, is a powerful tool for determining conformational preferences. Coupling constants, chemical shifts, and nuclear Overhauser effect (NOE) measurements can provide valuable information about the relative orientation of substituents and the overall ring conformation.
-
X-ray Crystallography: X-ray crystallography provides high-resolution structural information, offering a definitive picture of the molecule's conformation in the solid state. However, it is crucial to note that the conformation in the solid state may not perfectly reflect the conformation in solution.
-
Computational Methods: Computational methods, such as molecular mechanics (MM), molecular dynamics (MD), and density functional theory (DFT) calculations, can be employed to predict conformational energies and preferences. These methods allow the exploration of a wider range of conformations and provide valuable insights into the factors governing conformational equilibria.
Implications for Reactivity
The conformation of a trisubstituted cyclohexane significantly impacts its reactivity. The accessibility of substituents to reagents is directly influenced by their axial or equatorial orientation. A distorted, partially planar conformation can alter the steric environment around the substituents, influencing reaction rates and selectivity.
Conclusion: Towards a Deeper Understanding
The conformational analysis of trisubstituted cyclohexanes presents a fascinating challenge. While true planarity is unlikely, various factors can lead to conformations that deviate significantly from the standard chair form. Understanding these factors requires a multifaceted approach, combining experimental techniques like NMR and X-ray crystallography with powerful computational methods. This intricate interplay of steric and electronic effects highlights the complexity and richness of organic chemistry, reminding us that seemingly simple structural modifications can have profound impacts on molecular properties and behavior. Future research in this area will undoubtedly continue to refine our understanding of conformational preferences and their consequences for reactivity and physical properties. Further investigation into specific examples with defined substituents will allow for a more precise and predictive analysis of conformational behavior.
Latest Posts
Latest Posts
-
Compute The Cost Of Direct Labor Used For The Period
Mar 19, 2025
-
Which Of The Following Is A Current Asset
Mar 19, 2025
-
Important Characteristics Of Antimicrobic Drugs Include
Mar 19, 2025
-
All Amino Acids Have Two Ionizable Functional Groups
Mar 19, 2025
-
A Suggested Active Reading Strategy Is To
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Given The Planar Trisubstituted Cyclohexane Below . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
