Give The Iupac Name For The Following Compound:
Holbox
Mar 25, 2025 · 6 min read
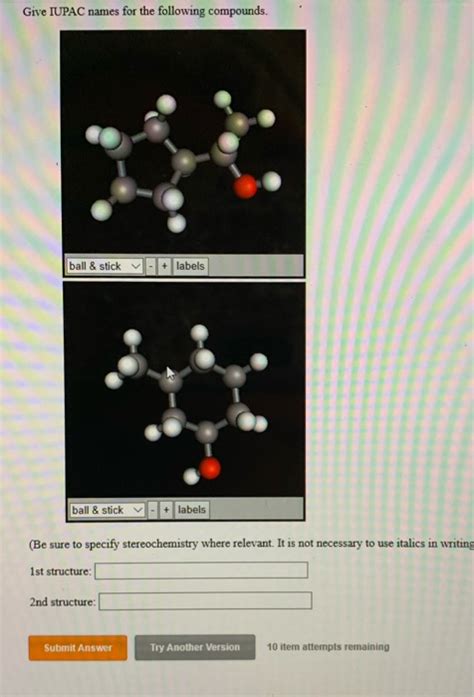
Table of Contents
- Give The Iupac Name For The Following Compound:
- Table of Contents
- Giving IUPAC Names to Organic Compounds: A Comprehensive Guide
- Understanding IUPAC Nomenclature: The Foundation
- Key Principles:
- Naming Alkanes: The Simplest Hydrocarbons
- Branched Alkanes: Introducing Substituents
- Naming Alkenes and Alkynes: Incorporating Unsaturation
- Handling Multiple Substituents and Complex Structures
- Functional Groups: Adding Complexity
- Alcohols (-OH):
- Aldehydes (-CHO):
- Ketones (C=O):
- Carboxylic Acids (-COOH):
- Amines (-NH₂):
- Ethers (R-O-R'):
- Combining Functional Groups and Prioritization
- Dealing with Complex Cyclic Structures
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Giving IUPAC Names to Organic Compounds: A Comprehensive Guide
Naming organic compounds might seem daunting at first, but with a systematic approach and understanding of IUPAC nomenclature rules, it becomes a manageable and even enjoyable task. This comprehensive guide will walk you through the process, equipping you with the knowledge to confidently name a wide range of organic molecules. We'll cover alkanes, alkenes, alkynes, alcohols, aldehydes, ketones, carboxylic acids, and more, demonstrating the principles through numerous examples.
Understanding IUPAC Nomenclature: The Foundation
The International Union of Pure and Applied Chemistry (IUPAC) established a standardized system for naming organic compounds to ensure clear and unambiguous communication within the scientific community. This system is based on a set of rules that consider the compound's structure, functional groups, and parent chain.
Key Principles:
-
Identifying the Parent Chain: This is the longest continuous carbon chain in the molecule. Numbering starts from the end closest to the highest priority functional group (discussed below).
-
Identifying Substituents: These are atoms or groups of atoms attached to the parent chain. They are named systematically and their position on the parent chain is indicated by a number.
-
Prioritizing Functional Groups: Different functional groups have different priorities in the naming system. Higher priority groups dictate the suffix of the name, while lower priority groups are named as prefixes.
-
Numbering the Carbon Chain: The parent chain is numbered to give the substituents the lowest possible numbers. If there's a tie, the next substituent's position is considered, and so on.
-
Alphabetical Ordering: Substituents are listed alphabetically, ignoring prefixes like di- or tri- (except for iso, sec, and tert).
Naming Alkanes: The Simplest Hydrocarbons
Alkanes are saturated hydrocarbons containing only single bonds. Their names follow a simple prefix-suffix system:
-
Prefix: Indicates the number of carbon atoms (meth- (1), eth- (2), prop- (3), but- (4), pent- (5), hex- (6), hept- (7), oct- (8), non- (9), dec- (10), etc.).
-
Suffix: "-ane" denotes an alkane.
Examples:
- CH₄: Methane
- CH₃CH₃: Ethane
- CH₃CH₂CH₃: Propane
- CH₃CH₂CH₂CH₃: Butane
- CH₃(CH₂)₃CH₃: Pentane
Branched Alkanes: Introducing Substituents
When alkanes have branches, we need to identify the longest continuous carbon chain as the parent chain and name the branches as substituents. The substituents are alkyl groups, named by replacing the "-ane" ending of the corresponding alkane with "-yl" (e.g., methyl, ethyl, propyl).
Example:
Consider the following compound:
CH₃
|
CH₃-CH-CH₂-CH₃
- Identify the parent chain: The longest continuous chain has four carbons, making it butane.
- Identify the substituent: A methyl group (CH₃) is attached to the second carbon.
- Number the chain: Numbering starts from the end closest to the substituent.
- Name the compound: 2-Methylbutane
Naming Alkenes and Alkynes: Incorporating Unsaturation
Alkenes contain at least one carbon-carbon double bond, and alkynes contain at least one carbon-carbon triple bond.
- Suffix: "-ene" for alkenes, "-yne" for alkynes.
Example (Alkene):
CH₂=CH-CH₂-CH₃
- Identify the parent chain: Four carbons – butene.
- Locate the double bond: The double bond is between carbons 1 and 2.
- Name the compound: 1-Butene (the position of the double bond is specified)
Example (Alkyne):
CH≡C-CH₂-CH₃
- Identify the parent chain: Four carbons – butyne.
- Locate the triple bond: The triple bond is between carbons 1 and 2.
- Name the compound: 1-Butyne
Handling Multiple Substituents and Complex Structures
When multiple substituents are present, they are listed alphabetically, and their positions are indicated by numbers. If the same substituent appears more than once, prefixes like di- (two), tri- (three), tetra- (four), etc., are used.
Example:
CH₃ CH₃
| |
CH₃-CH-CH-CH₂-CH₃
- Parent chain: Pentane (five carbons).
- Substituents: Two methyl groups.
- Positions: Methyl groups are at positions 2 and 3.
- Name: 2,3-Dimethylpentane
Functional Groups: Adding Complexity
Functional groups are specific groups of atoms within a molecule that determine its chemical properties and influence its IUPAC name. These groups often dictate the suffix of the compound's name.
Alcohols (-OH):
Alcohols contain a hydroxyl group (-OH). The suffix "-ol" is used. The position of the hydroxyl group is indicated by a number.
Example: CH₃CH₂CH₂OH → 1-Propanol
Aldehydes (-CHO):
Aldehydes contain a carbonyl group (-CHO) at the end of a carbon chain. The suffix "-al" is used. The aldehyde carbon is always carbon number 1.
Example: CH₃CH₂CHO → Propanal
Ketones (C=O):
Ketones have a carbonyl group (C=O) within the carbon chain. The suffix "-one" is used. The position of the carbonyl group is specified.
Example: CH₃COCH₃ → Propanone (or acetone, its common name)
Carboxylic Acids (-COOH):
Carboxylic acids have a carboxyl group (-COOH) at the end of a carbon chain. The suffix "-oic acid" is used. The carboxyl carbon is always carbon number 1.
Example: CH₃CH₂COOH → Propanoic acid
Amines (-NH₂):
Amines contain an amino group (-NH₂). The prefix "amino-" is used, indicating the position on the parent chain.
Example: CH₃CH(NH₂)CH₃ → 2-Aminopropane
Ethers (R-O-R'):
Ethers have an oxygen atom bonded to two alkyl or aryl groups. The names of the alkyl groups are listed alphabetically, followed by "ether".
Example: CH₃OCH₂CH₃ → Ethyl methyl ether
Combining Functional Groups and Prioritization
When multiple functional groups are present, a priority order determines which group dictates the suffix and which are prefixes. The order generally follows this hierarchy (from highest to lowest priority):
- Carboxylic acids
- Anhydrides
- Esters
- Amides
- Nitriles
- Aldehydes
- Ketones
- Alcohols
- Amines
- Alkenes
- Alkynes
- Alkanes
Example:
Consider a compound with both an alcohol and a ketone functional group:
O
||
CH₃-C-CH₂-CH₂-CH₂-OH
- Identify the parent chain: Pentane.
- Prioritize the functional groups: The ketone (C=O) has higher priority than the alcohol (-OH).
- Name the compound: 4-Hydroxy-2-pentanone (the alcohol is named as a prefix "hydroxy-").
Dealing with Complex Cyclic Structures
Cyclic compounds require additional considerations. The parent chain becomes the ring, and substituents are numbered accordingly. The ring's size is indicated by a prefix (cyclopropane, cyclobutane, cyclopentane, etc.).
Example:
CH₃
|
CH₂-CH₂
| |
CH₂-CH-CH₂
- Parent chain: Cyclohexane (six-membered ring).
- Substituent: Methyl group.
- Position: Methyl group at position 1 (or any position as cyclohexane is symmetrical).
- Name: Methylcyclohexane
Conclusion
Mastering IUPAC nomenclature requires practice and attention to detail. By systematically applying the rules outlined in this guide, you will develop the skills necessary to confidently name a wide variety of organic compounds, enhancing your understanding of organic chemistry and facilitating clear communication within the scientific community. Remember to always prioritize functional groups correctly and to adhere to the alphabetical order when listing substituents. The more you practice, the more intuitive the naming process will become. This is not just about memorization but about understanding the underlying logic of the system.
Latest Posts
Latest Posts
-
Brand Centric Demand Generation Focuses On
Mar 28, 2025
-
One Of Your Assignments At Work Is To Analyze
Mar 28, 2025
-
Draw The Product Of This Hydrogenation Reaction Ignore Inorganic Byproducts
Mar 28, 2025
-
You Sell Widgets For 80 Each
Mar 28, 2025
-
Draw The Lewis Structure For A Carbon Monosulfide Molecule
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Give The Iupac Name For The Following Compound: . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
