Draw The Lewis Structure For A Carbon Monosulfide Molecule
Holbox
Mar 28, 2025 · 6 min read
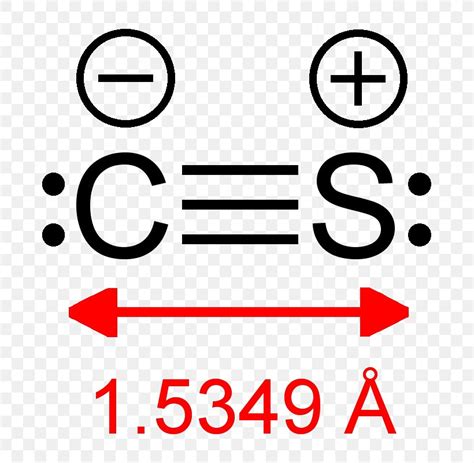
Table of Contents
- Draw The Lewis Structure For A Carbon Monosulfide Molecule
- Table of Contents
- Drawing the Lewis Structure for Carbon Monosulfide (CS): A Comprehensive Guide
- Understanding Lewis Structures
- Step-by-Step Construction of the CS Lewis Structure
- Step 1: Count Valence Electrons
- Step 2: Identify the Central Atom
- Step 3: Form Single Bonds
- Step 4: Complete Octet Rule (where possible)
- Step 5: Forming Multiple Bonds
- Step 6: Distribute Remaining Electrons
- Step 7: Final Lewis Structure of CS
- Exploring the Bonding in CS
- Properties of Carbon Monosulfide
- Applications of Carbon Monosulfide
- Further Considerations and Exceptions to the Octet Rule
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Drawing the Lewis Structure for Carbon Monosulfide (CS): A Comprehensive Guide
Carbon monosulfide (CS), a fascinating molecule with a triple bond, presents a compelling example for understanding Lewis structures. This guide will walk you through the step-by-step process of drawing its Lewis structure, exploring its bonding characteristics, and delving into its properties. We’ll also touch upon the applications of this intriguing molecule.
Understanding Lewis Structures
Before we dive into drawing the Lewis structure of CS, let's refresh our understanding of what a Lewis structure actually is. A Lewis structure, also known as a Lewis dot diagram, is a simplified representation of a molecule's valence electrons. It visually depicts the bonding between atoms and the lone pairs of electrons that may exist in the molecule. This representation is crucial in predicting the molecule's geometry, polarity, and reactivity. The fundamental principle behind a Lewis structure is the octet rule (with some exceptions), which states that atoms tend to gain, lose, or share electrons in order to have eight electrons in their valence shell.
Step-by-Step Construction of the CS Lewis Structure
Let's systematically construct the Lewis structure for carbon monosulfide, CS:
Step 1: Count Valence Electrons
First, we need to determine the total number of valence electrons in the molecule. Carbon (C) is in Group 14 of the periodic table and has four valence electrons. Sulfur (S) is in Group 16 and possesses six valence electrons. Therefore, the total number of valence electrons in CS is 4 + 6 = 10.
Step 2: Identify the Central Atom
In a diatomic molecule like CS, choosing a central atom isn’t strictly necessary, but it's helpful to designate one for consistency. Generally, the less electronegative atom is placed in the center. In this case, carbon is slightly less electronegative than sulfur, so we'll consider it as the central atom (although this choice has minimal impact on the final Lewis structure in this specific case).
Step 3: Form Single Bonds
We begin by forming a single bond between the carbon and sulfur atoms. A single bond consists of two electrons, so we use two of our ten valence electrons to create this bond: C-S. This leaves us with 8 electrons.
Step 4: Complete Octet Rule (where possible)
Next, we try to satisfy the octet rule for each atom by distributing the remaining electrons as lone pairs. However, we quickly find that we cannot achieve octets for both atoms using only single bonds. We have 8 electrons left, and if we distribute them as lone pairs (four electrons around each atom), neither atom achieves an octet.
Step 5: Forming Multiple Bonds
Since we can't satisfy the octet rule with single bonds, we need to form multiple bonds. We have eight electrons remaining. We can form a triple bond between carbon and sulfur, using six of the remaining electrons, leaving two electrons. This results in the structure: C≡S.
Step 6: Distribute Remaining Electrons
The remaining two electrons are added as a lone pair to the sulfur atom. Sulfur can accommodate more than eight electrons due to its ability to utilize d-orbitals (expanded octet). This is more common in sulfur than in carbon.
Step 7: Final Lewis Structure of CS
The final Lewis structure for carbon monosulfide (CS) is:
:C≡S:
The carbon atom forms a triple bond with the sulfur atom. The triple bond consists of three bonding pairs of electrons and the sulfur atom has one lone pair of electrons. The carbon atom doesn't possess any lone pairs of electrons in this case. This structure fulfills the need for 10 valence electrons and effectively satisfies the bonding requirements of both atoms.
Exploring the Bonding in CS
The triple bond in carbon monosulfide is a crucial feature of its bonding. This triple bond consists of one sigma (σ) bond and two pi (π) bonds. The sigma bond is formed by the head-on overlap of hybrid orbitals (sp hybridized in both carbon and sulfur), while the pi bonds are formed by the sideways overlap of p-orbitals. This strong triple bond accounts for the relatively high bond energy and short bond length of the CS molecule.
Properties of Carbon Monosulfide
Understanding the Lewis structure allows us to predict some of the properties of CS:
- Bond Order: The bond order is 3, indicating a strong triple bond.
- Bond Length: The bond length is relatively short due to the strong triple bond.
- Bond Energy: The bond energy is relatively high due to the strong triple bond.
- Polarity: While carbon and sulfur have different electronegativities, the molecule possesses a relatively small dipole moment due to the symmetrical distribution of electrons within the triple bond. However, there is a small difference in electronegativity, making it slightly polar, with the partial negative charge residing on the sulfur atom.
- Reactivity: The strong triple bond makes carbon monosulfide relatively unreactive compared to other similar molecules. However, it can participate in certain chemical reactions, particularly those involving oxidation-reduction or addition reactions.
- Physical State: Under standard conditions, CS is a colorless, toxic gas.
Applications of Carbon Monosulfide
Although not as widely used as some other molecules, CS finds applications in several areas:
- Chemical Synthesis: It is used as a reagent in organic chemistry and for the synthesis of other sulfur-containing compounds.
- Astrochemistry: CS is detected in interstellar space, playing a role in the study of star formation and the composition of interstellar clouds. Its presence in these environments provides valuable insight into the chemical processes occurring in the universe.
- Spectroscopy: Due to its unique spectral properties, CS is useful as a spectroscopic reference molecule, providing valuable data for calibrating instruments and understanding molecular vibrations.
Further Considerations and Exceptions to the Octet Rule
While the octet rule serves as a useful guideline for drawing Lewis structures, it's important to remember that there are exceptions. Elements in the third period and beyond can often expand their octet, accommodating more than eight electrons in their valence shell. Sulfur, in this case, displays this ability by accepting the lone pair of electrons.
Conclusion
Drawing the Lewis structure for carbon monosulfide effectively demonstrates the importance of understanding valence electrons, bonding, and the nuances of the octet rule. The resulting structure provides valuable insight into the molecule's properties, reactivity, and applications, from interstellar space to chemical synthesis. By following the step-by-step process outlined in this guide, you can confidently draw Lewis structures for a wide range of molecules, strengthening your understanding of chemical bonding. The understanding of Lewis structures opens doors to comprehending the intricate world of molecular behavior and its implications in various scientific fields. Remember, practice makes perfect, so continue practicing with different molecules to refine your skills and deeper your understanding.
Latest Posts
Latest Posts
-
The Advertising Director For A Guitar Manufacturer
Mar 31, 2025
-
Which Of The Following Relationships Is Correct
Mar 31, 2025
-
The Term Institutionalization Can Be Defined As
Mar 31, 2025
-
Anti Doping Policies Prior To The Mid 1980s Existed Largely To
Mar 31, 2025
-
In A States Pick 3 Lottery Game
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Draw The Lewis Structure For A Carbon Monosulfide Molecule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
