Give The Iupac Name For Each Compound
Holbox
Mar 14, 2025 · 7 min read
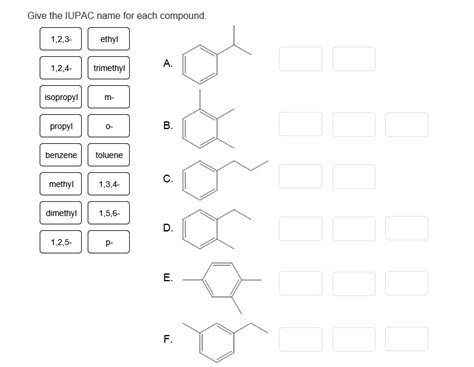
Table of Contents
Giving IUPAC Names to Organic Compounds: A Comprehensive Guide
Naming organic compounds might seem daunting at first, but with a systematic approach and understanding of IUPAC nomenclature rules, it becomes a manageable and even enjoyable task. The International Union of Pure and Applied Chemistry (IUPAC) provides a standardized system for naming organic molecules, ensuring clear and unambiguous communication among chemists worldwide. This comprehensive guide will delve into the intricacies of IUPAC nomenclature, providing examples and explanations to help you master this essential skill.
Understanding the Basics of IUPAC Nomenclature
Before diving into complex structures, let's establish a foundational understanding of the key principles:
1. Identifying the Parent Chain: The Foundation of IUPAC Names
The parent chain is the longest continuous carbon chain within the molecule. This forms the basis of the name. Consider this example:
CH3-CH2-CH2-CH2-CH3
The parent chain consists of five carbons, making it a pentane.
2. Identifying Substituents: Branches and Additions
Substituents are atoms or groups of atoms attached to the parent chain. Common substituents include alkyl groups (e.g., methyl, ethyl, propyl), halogens (e.g., chloro, bromo, iodo), and other functional groups (e.g., hydroxyl, carboxyl).
3. Numbering the Carbon Atoms: Establishing Position
The parent chain is numbered to assign positions to substituents. Numbering begins from the end that gives the substituents the lowest possible numbers. If there are multiple substituents, the lowest number is assigned to the substituent with alphabetical priority.
4. Alphabetical Ordering: Organizing Substituents
Substituents are listed alphabetically in the name, ignoring prefixes like di, tri, and tetra (except when determining alphabetical order among different substituents). Numbers indicating position precede the substituent names, separated by hyphens.
5. Using Prefixes for Multiple Substituents: Indicating Quantity
If a substituent appears more than once, prefixes such as di, tri, tetra, etc., are used. These prefixes are considered when alphabetizing substituents. Commas separate numbers, and hyphens separate numbers from words.
Naming Alkanes: The Simplest Hydrocarbons
Alkanes are saturated hydrocarbons with only single bonds between carbon atoms. Their IUPAC names follow a simple pattern:
- Meth- (1 carbon), Eth- (2 carbons), Prop- (3 carbons), But- (4 carbons), Pent- (5 carbons), Hex- (6 carbons), Hept- (7 carbons), Oct- (8 carbons), Non- (9 carbons), Dec- (10 carbons), and so on.
The suffix "-ane" denotes an alkane. For example:
- CH₄: Methane
- CH₃CH₃: Ethane
- CH₃CH₂CH₃: Propane
- CH₃CH₂CH₂CH₃: Butane
- CH₃CH₂CH₂CH₂CH₃: Pentane
Naming Branched Alkanes: Incorporating Substituents
When dealing with branched alkanes, follow these steps:
- Identify the longest continuous carbon chain: This forms the parent alkane.
- Identify the substituents: These are alkyl groups branching off the parent chain.
- Number the parent chain: Begin numbering from the end closest to the first substituent. If substituents are equidistant from both ends, prioritize the alphabetical order of the substituents.
- List the substituents alphabetically: Use prefixes like di, tri, tetra, etc., for multiple identical substituents. Remember to ignore these prefixes when alphabetizing, except when comparing different substituents.
- Combine the information: Write the name as a single word, with numbers and hyphens used to indicate positions of substituents.
Examples:
-
CH₃CH(CH₃)CH₂CH₃: 2-Methylbutane (The longest chain has four carbons – butane. A methyl group is on carbon 2).
-
CH₃CH₂CH(CH₃)CH₂CH₃: 3-Methylpentane (The longest chain has five carbons – pentane. A methyl group is on carbon 3).
-
CH₃C(CH₃)₂CH₃: 2,2-Dimethylpropane (The longest chain has three carbons – propane. Two methyl groups are on carbon 2).
-
CH₃CH(CH₂CH₃)CH₂CH₃: 3-Ethylpentane (The longest chain is five carbons long. An ethyl group is attached to carbon 3).
-
CH₃CH(CH₃)CH(CH₃)CH₃: 2,3-Dimethylbutane (Four carbon parent chain. Methyl groups at positions 2 and 3. Note that 2,3 is lower than 3,4).
Incorporating Halogens and other Functional Groups
Halogens (fluorine, chlorine, bromine, iodine) are named as fluoro, chloro, bromo, iodo respectively. These are treated as substituents and are included alphabetically in the name.
Examples:
- CH₃CH₂Cl: Chloroethane
- CH₃CHBrCH₃: 2-Bromopropane
- CH₂ClCH₂Cl: 1,2-Dichloroethane
- CH₃CH₂CH₂F: 1-Fluoropropane
Many other functional groups will significantly impact the IUPAC naming, changing the parent chain designation and even the suffix. More complex examples including alcohols, ketones, aldehydes, carboxylic acids and others will be discussed below.
Naming Alkenes and Alkynes: Unsaturated Hydrocarbons
Alkenes contain at least one carbon-carbon double bond, while alkynes contain at least one carbon-carbon triple bond.
Alkenes:
- Identify the longest continuous carbon chain containing the double bond: This is the parent chain.
- Number the carbon atoms: Start numbering from the end closest to the double bond. The double bond gets the lowest possible number.
- Indicate the position of the double bond: Use the lower number of the two carbons involved in the double bond.
- Use the suffix "-ene": This indicates the presence of a double bond.
Examples:
-
CH₂=CHCH₃: Propene (The double bond is between carbons 1 and 2; only the lower number, 1, is used). It is not 1-propene.
-
CH₃CH=CHCH₃: 2-Butene (The double bond is between carbons 2 and 3).
-
CH₃CH₂CH=CHCH₃: 2-Pentene
Alkynes:
Alkynes are named similarly to alkenes, but with the suffix "-yne" to indicate the presence of a triple bond.
Examples:
-
CH≡CH: Ethyne (Commonly known as acetylene).
-
CH₃C≡CH: Propyne
-
CH₃CH₂C≡CH: 1-Butyne
Naming Alcohols: The Hydroxyl Group (-OH)
Alcohols contain a hydroxyl group (-OH). The naming convention involves:
- Identify the longest continuous carbon chain containing the hydroxyl group.
- Number the carbon atoms: Start numbering from the end closest to the hydroxyl group.
- Replace the "-e" ending of the alkane with "-ol": This indicates the presence of the alcohol functional group.
- Use the number to indicate the position of the hydroxyl group.
Examples:
-
CH₃OH: Methanol
-
CH₃CH₂OH: Ethanol
-
CH₃CH₂CH₂OH: Propan-1-ol
-
CH₃CH(OH)CH₃: Propan-2-ol
Naming Ketones: The Carbonyl Group (C=O)
Ketones contain a carbonyl group (C=O) bonded to two alkyl or aryl groups.
- Identify the longest continuous carbon chain containing the carbonyl group.
- Number the carbon atoms: Start numbering from the end closest to the carbonyl group.
- Replace the "-e" ending of the alkane with "-one": This indicates a ketone.
- Use the number to indicate the position of the carbonyl group.
Examples:
-
CH₃COCH₃: Propan-2-one (Commonly known as acetone).
-
CH₃CH₂COCH₃: Butan-2-one
-
CH₃COCH₂CH₃: Butan-2-one (Same as above, as the carbonyl group is in the same position).
Naming Aldehydes: The Formyl Group (-CHO)
Aldehydes contain a formyl group (-CHO) at the end of a carbon chain.
- Identify the longest continuous carbon chain containing the formyl group.
- Number the carbon atoms: The carbon of the formyl group is always carbon 1.
- Replace the "-e" ending of the alkane with "-al": This indicates an aldehyde.
Examples:
-
HCHO: Methanal (Commonly known as formaldehyde).
-
CH₃CHO: Ethanal (Commonly known as acetaldehyde).
-
CH₃CH₂CHO: Propanal
Naming Carboxylic Acids: The Carboxyl Group (-COOH)
Carboxylic acids contain a carboxyl group (-COOH).
- Identify the longest continuous carbon chain containing the carboxyl group.
- Number the carbon atoms: The carbon of the carboxyl group is always carbon 1.
- Replace the "-e" ending of the alkane with "-oic acid": This indicates a carboxylic acid.
Examples:
-
HCOOH: Methanoic acid (Commonly known as formic acid).
-
CH₃COOH: Ethanoic acid (Commonly known as acetic acid).
-
CH₃CH₂COOH: Propanoic acid
More Complex Structures: Combining Rules
Many organic molecules possess multiple functional groups. In these cases, a hierarchy of functional groups determines the parent chain and suffix. The priority order generally follows the order presented above, with carboxylic acids taking precedence over other functional groups. Specific rules apply to prioritize functional groups and assign the correct IUPAC name for complex molecules containing different functional groups. This requires a more in-depth understanding of organic chemistry and advanced IUPAC nomenclature.
This comprehensive guide provides a strong foundation for naming a wide range of organic compounds. Remember that practice is key to mastering IUPAC nomenclature. The more examples you work through, the more comfortable you will become with applying these rules and accurately naming organic molecules. Consult additional resources and textbooks for further clarification on complex structures and functional groups. Understanding IUPAC nomenclature is crucial for effective communication in the field of organic chemistry.
Latest Posts
Latest Posts
-
What Is The Conjugate Base Of Nh3
Mar 14, 2025
-
Contingent Liabilities Must Be Recorded If
Mar 14, 2025
-
A Price Setter Company Will Use More
Mar 14, 2025
-
Integrity Of E Phi Requires Confirmation That The Data
Mar 14, 2025
-
Most Of The Work On Legislation In Congress Is Done
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about Give The Iupac Name For Each Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
