For The Diprotic Weak Acid H2a
Holbox
Mar 16, 2025 · 5 min read
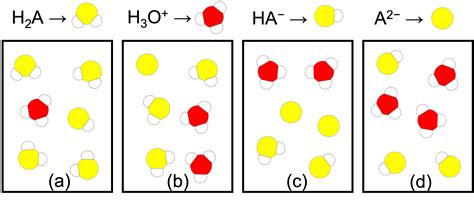
Table of Contents
- For The Diprotic Weak Acid H2a
- Table of Contents
- For the Diprotic Weak Acid H₂A: A Comprehensive Guide
- Understanding Diprotic Weak Acids
- Step 1: The First Ionization
- Step 2: The Second Ionization
- Calculating pH and pKa for H₂A
- Simplifying Assumptions:
- Approximation for Kₐ₁ >> Kₐ₂:
- The Significance of pKa:
- Buffers and Diprotic Acids
- Titration Curves of Diprotic Acids
- Applications of Diprotic Acids
- Advanced Considerations:
- Conclusion:
- Latest Posts
- Latest Posts
- Related Post
For the Diprotic Weak Acid H₂A: A Comprehensive Guide
Diprotic weak acids, like H₂A, present a fascinating challenge in chemistry. Understanding their behavior requires a deeper dive into equilibrium concepts than monoprotic acids. This comprehensive guide will delve into the intricacies of H₂A, exploring its ionization, calculations involving pH and pKa, and the applications of these concepts. We’ll also touch upon the practical significance of diprotic acids and their role in various fields.
Understanding Diprotic Weak Acids
A diprotic acid, unlike a monoprotic acid (like HCl), can donate two protons (H⁺) per molecule. The term "weak" indicates that the acid doesn't completely dissociate in water. Instead, it establishes an equilibrium between the undissociated acid and its conjugate bases. For H₂A, this ionization occurs in two distinct steps:
Step 1: The First Ionization
H₂A(aq) ⇌ H⁺(aq) + HA⁻(aq) Kₐ₁
This step represents the loss of the first proton. The equilibrium constant, Kₐ₁, represents the strength of the acid in this first dissociation. A smaller Kₐ₁ value indicates a weaker acid in this first step.
Step 2: The Second Ionization
HA⁻(aq) ⇌ H⁺(aq) + A²⁻(aq) Kₐ₂
Here, the conjugate base from the first ionization (HA⁻) loses its second proton. Kₐ₂ is the equilibrium constant for this second step. Generally, Kₐ₂ is significantly smaller than Kₐ₁. This is because it's more difficult to remove a proton from a negatively charged species (HA⁻) than from a neutral species (H₂A).
Calculating pH and pKa for H₂A
Calculating the pH of a diprotic acid solution is more complex than for a monoprotic acid because it involves two equilibrium expressions. However, several simplifying assumptions can be made, depending on the relative magnitudes of Kₐ₁ and Kₐ₂.
Simplifying Assumptions:
-
If Kₐ₁ >> Kₐ₂: This is the most common scenario. The second ionization contributes negligibly to the overall [H⁺] concentration. The pH can be approximated by considering only the first ionization. This simplifies the calculation significantly.
-
If Kₐ₁ and Kₐ₂ are close: This scenario requires a more complex iterative calculation or the use of numerical methods to solve the simultaneous equilibrium equations. Software or advanced calculators are often helpful in these situations.
Approximation for Kₐ₁ >> Kₐ₂:
-
Consider only the first ionization: Use the ICE (Initial, Change, Equilibrium) table to set up the equilibrium expression for Kₐ₁:
Species Initial Change Equilibrium H₂A C -x C - x H⁺ 0 +x x HA⁻ 0 +x x Where 'C' is the initial concentration of H₂A.
-
Solve for x: Kₐ₁ = (x²)/(C - x). Often, the 'x' in the denominator can be neglected if Kₐ₁ is small compared to C (simplifying the quadratic equation). This gives x ≈ √(Kₐ₁C).
-
Calculate pH: pH = -log₁₀(x)
The Significance of pKa:
The pKa values (-log₁₀(Ka)) provide crucial insights into the acidity of H₂A. A lower pKa value indicates a stronger acid at that ionization step. The difference between pKa₁ and pKa₂ reflects the relative ease of removing the first versus the second proton.
Buffers and Diprotic Acids
Diprotic acids can form two different buffer solutions:
-
H₂A/HA⁻ buffer: This buffer resists changes in pH around a range close to pKa₁.
-
HA⁻/A²⁻ buffer: This buffer resists changes in pH around a range close to pKa₂.
The Henderson-Hasselbalch equation can be used to calculate the pH of these buffers:
pH = pKa + log₁₀([conjugate base]/[acid])
For the H₂A/HA⁻ buffer, the equation becomes:
pH ≈ pKa₁ + log₁₀([HA⁻]/[H₂A])
And for the HA⁻/A²⁻ buffer:
pH ≈ pKa₂ + log₁₀([A²⁻]/[HA⁻])
Titration Curves of Diprotic Acids
Titrating a diprotic acid with a strong base yields a titration curve with two equivalence points. These points correspond to the complete neutralization of the first and second protons. The curve shows two distinct buffering regions, one around each pKa value. The midpoint of each buffering region corresponds to the pKa value. Analyzing the titration curve provides valuable information about the pKa values and the concentrations of the acid and its conjugate bases.
Applications of Diprotic Acids
Diprotic acids play a crucial role in various areas:
-
Biological Systems: Many important biological molecules are diprotic acids, including amino acids (with carboxyl and amino groups) and phosphoric acid (a key component of DNA and ATP). Their ability to act as buffers is essential for maintaining the pH stability of biological systems.
-
Industrial Processes: Diprotic acids find applications in various industrial processes, including:
-
Food Industry: Ascorbic acid (Vitamin C) is a diprotic acid used as an antioxidant and preservative. Citric acid, another diprotic acid, is used as a flavoring agent and acidulant.
-
Chemical Synthesis: Many diprotic acids serve as reactants or catalysts in chemical synthesis.
-
Water Treatment: Certain diprotic acids are used in water treatment for pH adjustment and metal ion removal.
-
-
Analytical Chemistry: Diprotic acids are used as standards in acid-base titrations and as components in buffer solutions used in analytical methods.
Advanced Considerations:
-
Activity Coefficients: At higher concentrations, the activity of ions deviates from their concentration. Activity coefficients need to be considered for more accurate calculations.
-
Ionic Strength: The presence of other ions in the solution affects the activity coefficients. The ionic strength of the solution should be considered for precise pH calculations.
-
Temperature Dependence: The Kₐ values are temperature-dependent. The calculations need to be adjusted if the temperature differs significantly from the standard temperature (25°C).
Conclusion:
Diprotic weak acids like H₂A demonstrate a complex yet fascinating equilibrium behavior. While the calculations can appear challenging at first, understanding the principles of ionization, equilibrium constants, and the simplifying assumptions allows for accurate predictions of pH and the behavior of these acids in various solutions. Their importance in biological systems, industrial processes, and analytical chemistry highlights their significance in numerous fields. This comprehensive guide provides a strong foundation for understanding the chemistry of diprotic weak acids and their diverse applications. By grasping the core concepts outlined here, you'll be equipped to tackle more complex problems and gain a deeper appreciation for the intricacies of acid-base chemistry.
Latest Posts
Latest Posts
-
Overapplied Manufacturing Overhead Would Result If
Mar 17, 2025
-
All Of The Following Accurately Describe Lockout Tags Except
Mar 17, 2025
-
What Is The Narrowest Definition Of The Number 6
Mar 17, 2025
-
Strategic Implementation Is Thought To Be
Mar 17, 2025
-
Laker Company Reported The Following January
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about For The Diprotic Weak Acid H2a . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
