For The Dehydrohalogenation E2 Reaction Shown
Holbox
Mar 20, 2025 · 7 min read
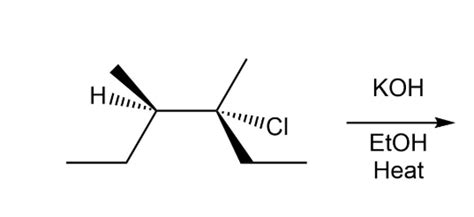
Table of Contents
Dehydrohalogenation E2 Reaction: A Comprehensive Guide
The E2 reaction, or elimination bimolecular reaction, is a crucial organic chemistry reaction that involves the removal of a hydrogen halide (HX) from a substrate, typically an alkyl halide, to form an alkene. This reaction is characterized by its concerted mechanism, meaning the bond breaking and bond formation occur simultaneously. Understanding the nuances of the E2 reaction, particularly concerning its stereochemistry and regiochemistry, is vital for synthetic organic chemists. This comprehensive guide delves deep into the dehydrohalogenation E2 reaction, exploring its mechanism, stereochemistry, regiochemistry, reaction conditions, and applications.
The Mechanism of the E2 Reaction
The E2 reaction proceeds via a single-step, concerted mechanism. This means that the breaking of the C-H and C-X bonds and the formation of the C=C pi bond all occur simultaneously in one transition state. The reaction requires a strong base to abstract a proton (H+) from a carbon atom adjacent to the carbon atom bearing the leaving group (X). This simultaneous process leads to several key characteristics:
Concerted Nature and Transition State
The concerted nature is a defining feature of the E2 reaction. The transition state involves partial bond breaking and formation. The base interacts with the β-hydrogen (the hydrogen on the carbon adjacent to the carbon bearing the leaving group), while the leaving group begins to depart. Simultaneously, the pi bond begins to form between the α-carbon (the carbon bearing the leaving group) and the β-carbon. This simultaneous process requires a specific spatial arrangement of the reactants, as detailed in the stereochemistry section.
Role of the Base
The strength of the base significantly influences the rate of the E2 reaction. Strong bases, such as potassium tert-butoxide (t-BuOK), sodium ethoxide (NaOEt), and potassium hydroxide (KOH), are necessary because they readily abstract the β-hydrogen. Weaker bases will not be effective, and other reaction pathways, such as SN2 substitution, may dominate. The choice of base also impacts regioselectivity, as we'll discuss later.
Leaving Group Ability
The leaving group's ability to depart also significantly impacts the reaction rate. Good leaving groups, such as halides (I⁻ > Br⁻ > Cl⁻ > F⁻), tosylates (OTs), and mesylates (OMs), readily depart, facilitating the E2 reaction. Poor leaving groups, on the other hand, hinder the process, often leading to competing reactions.
Stereochemistry of the E2 Reaction: Anti-Periplanar Geometry
The E2 reaction exhibits a strict stereochemical requirement: the β-hydrogen and the leaving group must be anti-periplanar. This means they must be on opposite sides of the molecule and in the same plane. This anti-periplanar geometry is crucial for the concerted mechanism to occur efficiently. The orbitals involved in bond breaking and bond formation must have the correct alignment for optimal overlap, leading to a low-energy transition state.
Conformation and its Impact on E2 Reaction
Achieving the anti-periplanar geometry often requires the molecule to adopt a specific conformation. For instance, in cyclohexane systems, the reaction will proceed more favorably when the leaving group and the β-hydrogen are both in the axial positions. This conformation places them in the anti-periplanar orientation, allowing for a smooth E2 reaction. If these groups are equatorial, the reaction is either significantly slower or doesn't occur at all.
Understanding the Consequences of Anti-Periplanar Geometry
The anti-periplanar geometry dictates the stereochemistry of the alkene product. If the starting material is chiral, the E2 reaction can produce either a cis or trans alkene, depending on the relative positions of the substituents. This is a critical consideration in planning organic syntheses. Careful analysis of the starting material's conformation is necessary to predict the stereochemistry of the alkene product.
Regiochemistry of the E2 Reaction: Zaitsev's Rule
When the substrate contains multiple β-hydrogens, the regiochemistry of the E2 reaction—the position of the double bond in the product—is governed by Zaitsev's rule. Zaitsev's rule states that the major product will be the most substituted alkene (the alkene with the most alkyl groups attached to the double bond carbons). This is because the most substituted alkene is generally more stable due to hyperconjugation.
Exceptions to Zaitsev's Rule
While Zaitsev's rule is a reliable predictor, there are exceptions. Using a bulky base, like potassium tert-butoxide, can favor the formation of the less substituted alkene (Hofmann product). The bulky base sterically hinders the approach to the more substituted β-hydrogen, making the abstraction of the less hindered β-hydrogen more favorable. This is a valuable tool in synthetic organic chemistry for selectively generating less substituted alkenes.
Understanding the Influence of Base Sterics
The steric hindrance exerted by the base plays a crucial role in determining the regioselectivity of the E2 reaction. Smaller bases preferentially abstract the most accessible β-hydrogen, leading to the Zaitsev product. Larger bases, however, selectively abstract the less hindered β-hydrogen, leading to the Hofmann product. This allows chemists to control the regiochemistry of the reaction by selecting the appropriate base.
Reaction Conditions for the E2 Reaction
Optimizing the reaction conditions is crucial for achieving high yields and selectivity in E2 reactions.
Solvent Selection
The choice of solvent plays a significant role. A polar aprotic solvent, such as dimethyl sulfoxide (DMSO) or dimethylformamide (DMF), is generally preferred. These solvents solvate the cation effectively, increasing the base's reactivity without solvating the base too much, thus maintaining its nucleophilicity.
Temperature
The reaction temperature also needs consideration. Higher temperatures generally favor the E2 reaction, providing enough energy to overcome the activation barrier. However, excessively high temperatures can lead to side reactions or decomposition of the reactants.
Substrate Concentration
The concentration of the substrate impacts the reaction rate. Higher substrate concentration increases the chances of successful collisions between the base and the substrate.
Applications of the E2 Reaction
The E2 reaction is a widely used synthetic tool in organic chemistry, with numerous applications in diverse areas.
Synthesis of Alkenes
The most prominent application is the synthesis of alkenes from alkyl halides. The reaction provides a straightforward and efficient method for creating carbon-carbon double bonds, which are essential structural elements in many organic molecules. The regio- and stereoselectivity can be fine-tuned by choosing the appropriate base and reaction conditions.
Synthesis of Cyclic Compounds
The E2 reaction is also employed in the synthesis of cyclic compounds. Intramolecular E2 reactions, where the base abstracts a proton from a carbon atom within the same molecule, leading to the formation of a cyclic alkene. This is particularly useful in the synthesis of medium and large rings.
Preparation of Conjugated Dienes
The E2 reaction can produce conjugated dienes, compounds with alternating single and double bonds. This is particularly useful in the synthesis of various natural products and biologically active molecules.
Advanced Considerations and Variations
Competition with SN2 Reactions
The E2 reaction often competes with the SN2 reaction, especially when the substrate is primary or secondary. The factors influencing this competition include the base's strength, steric hindrance, and the leaving group's ability. Strong, bulky bases favor E2, while strong, less sterically hindered bases favor SN2.
Syn Elimination
While less common than anti-elimination, syn-elimination can occur in specific cases, such as with certain cyclic systems or substrates bearing strong electron-withdrawing groups.
Stereoselective E2 Reactions
By carefully controlling the stereochemistry of the starting material and the reaction conditions, chemists can achieve high degrees of stereoselectivity in E2 reactions, generating a specific isomer of the alkene product. This precision is essential in many organic synthesis pathways.
Conclusion
The E2 reaction is a fundamental and versatile tool in organic synthesis, offering a straightforward approach to alkene synthesis with precise control over regio- and stereochemistry. Understanding its mechanism, stereochemical requirements, and the influence of reaction conditions is vital for any organic chemist. The ability to manipulate the reaction through careful choice of base, solvent, and temperature provides a powerful method for constructing various complex molecules, with applications spanning diverse fields from pharmaceuticals to materials science. Continuous research in this area continues to unveil new aspects of the E2 reaction, further expanding its utility in organic synthesis.
Latest Posts
Latest Posts
-
Which Solutions Showed The Greatest Change In Ph Why
Mar 21, 2025
-
Which Of The Following Terms Measures Resource And Waste Impacts
Mar 21, 2025
-
List The Following Events In The Correct Order
Mar 21, 2025
-
A Service Sink Should Be Used To
Mar 21, 2025
-
In Each Reaction Box Place The Best Reagent And Conditions
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about For The Dehydrohalogenation E2 Reaction Shown . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
