Which Solutions Showed The Greatest Change In Ph Why
Holbox
Mar 21, 2025 · 6 min read
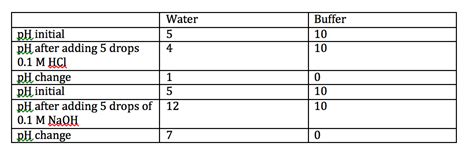
Table of Contents
- Which Solutions Showed The Greatest Change In Ph Why
- Table of Contents
- Which Solutions Showed the Greatest Change in pH? Why?
- Strong Acids and Bases: The Dramatic pH Shifters
- Hydrochloric Acid (HCl): A Case Study
- Sodium Hydroxide (NaOH): Another Extreme Example
- Why the Extreme Changes?
- Weak Acids and Bases: More Subtle pH Shifts
- Buffer Solutions: Resisting pH Changes
- Why the Subtle Changes?
- Factors Influencing pH Change Magnitude
- Specific Examples of Significant pH Changes
- Measuring pH Changes: Indicators and Instruments
- Conclusion: Understanding the pH Dance
- Latest Posts
- Latest Posts
- Related Post
Which Solutions Showed the Greatest Change in pH? Why?
Understanding pH changes is crucial in various fields, from chemistry and biology to environmental science and medicine. pH, a measure of the acidity or alkalinity of a solution, is determined by the concentration of hydrogen ions (H⁺). A change in pH signifies a shift in this concentration, indicating a chemical reaction or process has taken place. This article delves into the solutions that exhibit the most significant pH alterations and the underlying reasons for these changes.
Strong Acids and Bases: The Dramatic pH Shifters
The most dramatic pH changes are observed when strong acids or bases are added to water or other solutions. Strong acids, like hydrochloric acid (HCl) and sulfuric acid (H₂SO₄), completely dissociate in water, releasing a high concentration of H⁺ ions. This drastically lowers the pH, making the solution highly acidic. Conversely, strong bases, such as sodium hydroxide (NaOH) and potassium hydroxide (KOH), completely dissociate, releasing a high concentration of hydroxide ions (OH⁻). This drastically increases the pH, making the solution highly alkaline.
Hydrochloric Acid (HCl): A Case Study
Adding even a small amount of concentrated HCl to water causes a massive drop in pH. This is because HCl readily dissociates into H⁺ and Cl⁻ ions. The abundance of H⁺ ions overwhelms the equilibrium, significantly lowering the pH. The magnitude of the change depends on the concentration of HCl and the volume of water. A concentrated solution of HCl will cause a far more dramatic pH shift than a dilute solution.
Sodium Hydroxide (NaOH): Another Extreme Example
Similarly, adding NaOH to water leads to a substantial increase in pH. NaOH dissociates into Na⁺ and OH⁻ ions. The high concentration of OH⁻ ions reacts with H⁺ ions present in water, reducing their concentration and dramatically increasing the pH. Again, the concentration of NaOH directly impacts the magnitude of the pH change.
Why the Extreme Changes?
The extreme pH changes observed with strong acids and bases stem from their complete dissociation. Unlike weak acids and bases, which only partially dissociate, strong acids and bases release a proportionally larger number of H⁺ or OH⁻ ions, leading to a more pronounced effect on the pH. This complete ionization is a defining characteristic of strong acids and bases.
Weak Acids and Bases: More Subtle pH Shifts
Weak acids and bases, such as acetic acid (CH₃COOH) and ammonia (NH₃), only partially dissociate in water. This means that only a fraction of the acid or base molecules break down into ions. As a result, the change in pH is less dramatic compared to strong acids and bases. The extent of dissociation depends on the acid or base dissociation constant (Ka or Kb). A higher Ka or Kb indicates a stronger weak acid or base, leading to a greater, but still less dramatic, change in pH.
Buffer Solutions: Resisting pH Changes
Buffer solutions are particularly interesting in their response to pH changes. These solutions are composed of a weak acid and its conjugate base (or a weak base and its conjugate acid). They resist changes in pH when small amounts of strong acid or base are added. This is because the weak acid/base and its conjugate can neutralize the added H⁺ or OH⁻ ions, minimizing the overall impact on pH. The effectiveness of a buffer is determined by its buffer capacity, which depends on the concentrations of the weak acid and its conjugate base.
Why the Subtle Changes?
The relatively smaller pH changes with weak acids and bases arise from their incomplete dissociation. The equilibrium between undissociated molecules and ions prevents a significant shift in H⁺ or OH⁻ ion concentration, resulting in a more moderate pH alteration.
Factors Influencing pH Change Magnitude
Several factors influence the magnitude of pH change:
-
Concentration: Higher concentrations of acids or bases lead to greater pH changes. This is directly related to the number of H⁺ or OH⁻ ions released into the solution.
-
Volume: The volume of the solution also plays a role. Adding the same amount of acid or base to a smaller volume will result in a more significant pH change compared to adding it to a larger volume.
-
Temperature: Temperature can affect the dissociation constant of weak acids and bases, influencing their degree of ionization and therefore the pH change.
-
Presence of other ions: The presence of other ions in the solution can influence the activity of H⁺ and OH⁻ ions, affecting the pH change. This is particularly relevant in solutions with high ionic strength.
Specific Examples of Significant pH Changes
Let's consider some specific scenarios demonstrating significant pH shifts:
-
Acid Rain: Acid rain, caused by the release of sulfur dioxide and nitrogen oxides into the atmosphere, dramatically lowers the pH of lakes and rivers. This can have devastating effects on aquatic life.
-
Ocean Acidification: The absorption of excess carbon dioxide by the oceans leads to the formation of carbonic acid, lowering the pH of seawater. This process threatens marine organisms with calcium carbonate shells or skeletons.
-
Digestion: The human digestive system utilizes pH changes to facilitate digestion. The stomach, with its highly acidic environment (low pH), plays a crucial role in breaking down food, while the intestines maintain a more alkaline pH.
-
Industrial Processes: Many industrial processes involve controlling pH for optimal reaction conditions. For example, the production of certain chemicals or the treatment of wastewater often requires precise pH adjustments.
Measuring pH Changes: Indicators and Instruments
The change in pH can be measured using various methods:
-
pH Indicators: These are substances that change color depending on the pH of the solution. Litmus paper, for example, is a common pH indicator.
-
pH Meters: Electronic instruments, called pH meters, provide more precise pH measurements. They measure the potential difference between a reference electrode and a pH-sensitive electrode, which is directly related to the pH of the solution.
Conclusion: Understanding the pH Dance
The magnitude of pH change in a solution is governed by the strength and concentration of the acid or base added, as well as other factors like temperature and the presence of other ions. Strong acids and bases cause dramatic pH shifts due to their complete dissociation, while weak acids and bases produce more subtle changes. Understanding these dynamics is crucial in many scientific and practical applications, from environmental monitoring to industrial processes and biological systems. By carefully controlling pH, we can optimize chemical reactions, protect ecosystems, and maintain the health of living organisms. The pH scale is a powerful tool for understanding and controlling the chemical environment, influencing countless processes that shape our world.
Latest Posts
Latest Posts
-
The Strategy Making Strategy Executing Process
Mar 28, 2025
-
Joe If Another Phone Service Provider Enters The Market
Mar 28, 2025
-
Pal Cadaver Axial Skeleton Vertebral Column Lab Practical Question 9
Mar 28, 2025
-
Labor Efficiency Is Measured By The
Mar 28, 2025
-
A Differentiation Strategy Works Best When
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Which Solutions Showed The Greatest Change In Ph Why . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
