Enter The Orbital Diagram For The Ion Cd2+ .
Holbox
Mar 18, 2025 · 6 min read
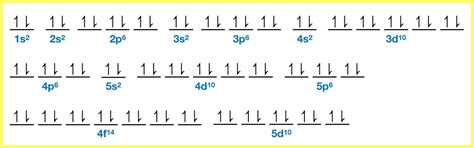
Table of Contents
Entering the Orbital Diagram for the Cd²⁺ Ion: A Comprehensive Guide
Understanding electron configurations and orbital diagrams is crucial in chemistry, providing insights into an atom's or ion's chemical behavior. This article delves into the process of constructing the orbital diagram for the cadmium(II) ion (Cd²⁺), explaining the underlying principles and offering a step-by-step approach. We'll explore the nuances of electron filling, Hund's rule, and the implications of ionization on the electronic structure.
Understanding Electronic Configuration and Orbital Diagrams
Before diving into the Cd²⁺ ion, let's refresh our understanding of electronic configurations and orbital diagrams. The electronic configuration describes the arrangement of electrons within an atom's energy levels and sublevels. It follows the Aufbau principle, which dictates that electrons fill orbitals starting from the lowest energy level to the highest. The Pauli Exclusion Principle states that each orbital can hold a maximum of two electrons with opposite spins (represented by arrows pointing up ↑ and down ↓). Hund's rule adds that within a subshell, electrons will individually occupy each orbital before pairing up in the same orbital.
An orbital diagram is a visual representation of the electronic configuration. It uses boxes to represent orbitals and arrows to represent electrons, clearly showing electron pairing and spin. For instance, the electronic configuration of carbon (C) is 1s²2s²2p², and its orbital diagram shows two electrons in the 1s orbital, two in the 2s orbital, and two in the 2p orbitals, with each 2p orbital initially occupied by a single electron before pairing occurs.
The Electronic Configuration of Neutral Cadmium (Cd)
Cadmium (Cd) has an atomic number of 48, meaning it has 48 electrons in its neutral state. To determine its electronic configuration, we follow the Aufbau principle and fill orbitals according to their energy levels:
1s², 2s², 2p⁶, 3s², 3p⁶, 4s², 3d¹⁰, 4p⁶, 5s², 4d¹⁰
This configuration can be shortened using the noble gas notation, representing the filled inner shells with the symbol of the preceding noble gas (Krypton, Kr):
[Kr] 5s² 4d¹⁰
Ionization and the Formation of Cd²⁺
The Cd²⁺ ion is formed when a neutral cadmium atom loses two electrons. These electrons are removed from the outermost energy levels, following the general rule that electrons are removed from the highest principal quantum number (n) first, and then from the subshells within that level (following the order s, p, d, f).
In cadmium, the outermost electrons are located in the 5s orbital. Therefore, when cadmium loses two electrons to form Cd²⁺, those two electrons are removed from the 5s orbital.
Constructing the Orbital Diagram for Cd²⁺
With the two 5s electrons removed, the electronic configuration of Cd²⁺ becomes:
[Kr] 4d¹⁰
Now, let's construct the orbital diagram:
The 4d subshell contains five orbitals (4d<sub>xy</sub>, 4d<sub>xz</sub>, 4d<sub>yz</sub>, 4d<sub>x²-y²</sub>, 4d<sub>z²</sub>). Since there are ten electrons in the 4d subshell, each of these five orbitals will be completely filled with two electrons each (one spin up and one spin down).
Orbital Diagram for Cd²⁺:
4d: ↑↓ ↑↓ ↑↓ ↑↓ ↑↓
This diagram illustrates that all five 4d orbitals are completely filled with electron pairs, signifying a stable electronic configuration for the Cd²⁺ ion. The absence of unpaired electrons contributes to the relatively low reactivity of Cd²⁺ compared to ions with unpaired electrons.
Implications of the Cd²⁺ Electronic Configuration
The filled 4d subshell in Cd²⁺ has significant implications for its chemical properties:
-
Diamagnetism: Because all electrons are paired, Cd²⁺ is diamagnetic, meaning it is not attracted to a magnetic field. This contrasts with paramagnetic substances that have unpaired electrons and are attracted to a magnetic field.
-
Stability: The completely filled d subshell contributes to the overall stability of the Cd²⁺ ion. This stability influences its reactivity and the types of chemical bonds it can form.
-
Coordination Chemistry: Cd²⁺ is a common ion in coordination chemistry, forming complexes with various ligands. The filled d orbitals do not directly participate in the bonding interactions in many of these complexes, but the overall charge and size of the ion play crucial roles in coordination number and geometry.
-
Applications: The stability and chemical properties of Cd²⁺ have led to its use in various applications, including batteries, pigments, and catalysts (although environmental concerns related to cadmium are now leading to a search for alternatives).
Comparison to other d-block ions
It is instructive to compare the Cd²⁺ ion to other divalent ions of the d-block elements. For example, Zn²⁺ ([Ar] 3d¹⁰) also has a fully filled d-subshell, exhibiting similar diamagnetic properties and relatively low reactivity. However, ions like Fe²⁺ ([Ar] 3d⁶) or Cu²⁺ ([Ar] 3d⁹) possess unpaired electrons, leading to paramagnetism and different chemical behaviors. These differences highlight how the electronic configuration dictates the properties of ions.
Advanced Concepts and Further Exploration
The orbital diagram for Cd²⁺, while seemingly simple, opens the door to a deeper understanding of atomic structure and chemical bonding. Exploring these advanced concepts can enhance your grasp of the topic:
-
Relativistic effects: In heavier atoms like cadmium, relativistic effects become increasingly important. These effects influence the energies of electron orbitals and can slightly alter the expected electronic configuration. While not significantly impacting the Cd²⁺ orbital diagram in this basic explanation, it is a noteworthy phenomenon for a more advanced understanding.
-
Crystal Field Theory: This theory explores the splitting of d orbitals in transition metal complexes, which significantly impacts their spectroscopic properties and reactivity. Understanding crystal field theory allows for a more detailed analysis of the behavior of Cd²⁺ in coordination complexes.
-
Ligand Field Theory: Building on Crystal Field Theory, Ligand Field Theory incorporates the covalent aspects of metal-ligand bonding, providing a more nuanced description of bonding interactions in transition metal complexes. Understanding ligand field theory is crucial for accurately predicting the properties of Cd²⁺ complexes.
Conclusion
Creating the orbital diagram for Cd²⁺ involves understanding electronic configurations, ionization processes, and the principles governing electron arrangement. The resulting diagram reveals a completely filled 4d subshell, leading to diamagnetism and relatively low reactivity. This seemingly simple exercise provides a foundation for deeper exploration into advanced concepts within inorganic chemistry. By appreciating the detailed electron arrangement, we gain valuable insight into the properties and behavior of this important ion, highlighting the fundamental connection between electronic structure and chemical reactivity. Remember that this knowledge is not only crucial for understanding the Cd²⁺ ion itself, but also serves as a stepping stone to more advanced concepts in chemistry.
Latest Posts
Latest Posts
-
What Is The Product Of This Reaction
Mar 18, 2025
-
Record The Entry To Close The Dividends Account
Mar 18, 2025
-
Which Of The Following Best Describes The Operational Period Briefing
Mar 18, 2025
-
The Second Largest Number Of Pacs Are Those Associated With
Mar 18, 2025
-
The Accompanying Graph Represents Haydens Fro Yo
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Enter The Orbital Diagram For The Ion Cd2+ . . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
