Enough Of A Monoprotic Acid Is Dissolved In Water
Holbox
Mar 16, 2025 · 6 min read
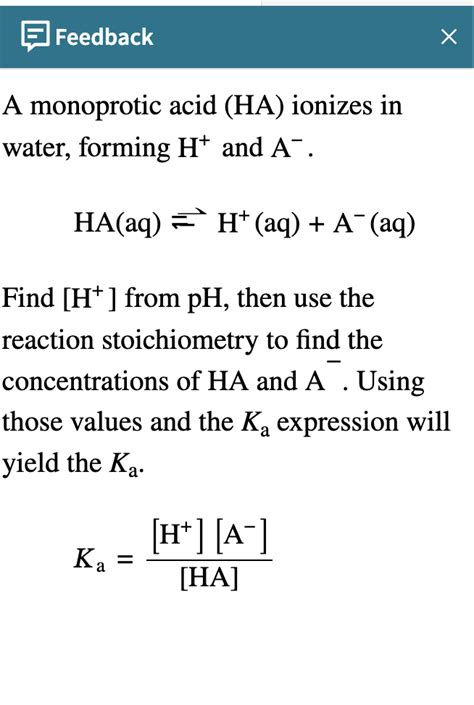
Table of Contents
Enough of a Monoprotic Acid is Dissolved in Water: A Deep Dive into Acid-Base Chemistry
Understanding the behavior of acids when dissolved in water is fundamental to chemistry. This article delves into the specifics of dissolving a monoprotic acid in water, exploring the equilibrium established, the factors influencing its strength, and the calculations involved in determining its concentration and pH. We'll cover various aspects, from basic definitions to more complex scenarios, making this a comprehensive guide for students and enthusiasts alike.
What is a Monoprotic Acid?
A monoprotic acid is an acid that can donate only one proton (H⁺) per molecule in an aqueous solution. This contrasts with polyprotic acids, such as sulfuric acid (H₂SO₄), which can donate more than one proton. Examples of common monoprotic acids include:
- Hydrochloric acid (HCl): A strong acid completely dissociating in water.
- Nitric acid (HNO₃): Another strong acid, fully dissociating in solution.
- Acetic acid (CH₃COOH): A weak acid, only partially dissociating in water.
- Formic acid (HCOOH): A weak acid similar to acetic acid in its behavior.
The key characteristic distinguishing monoprotic acids is their single acidic proton. This simplifies many calculations and allows for a straightforward understanding of their behavior in solution.
Dissolving a Monoprotic Acid in Water: The Equilibrium
When a monoprotic acid (HA) is dissolved in water, it undergoes a reversible reaction, establishing an equilibrium between the undissociated acid and its conjugate base (A⁻) and hydronium ions (H₃O⁺):
HA(aq) + H₂O(l) ⇌ H₃O⁺(aq) + A⁻(aq)
This equilibrium is governed by the acid dissociation constant (Ka), a measure of the acid's strength. A higher Ka value indicates a stronger acid, meaning it dissociates more readily. The Ka is defined as:
Ka = [H₃O⁺][A⁻] / [HA]
where the bracketed values represent the equilibrium concentrations of the respective species. The equilibrium expression shows that the product of the concentrations of the hydronium ions and the conjugate base, divided by the concentration of the undissociated acid, equals the acid dissociation constant.
Strong vs. Weak Monoprotic Acids
The extent of dissociation significantly differs between strong and weak monoprotic acids:
-
Strong Acids: These acids essentially completely dissociate in water. This means that the equilibrium lies far to the right, and the concentration of the undissociated acid ([HA]) is negligible. Therefore, for a strong monoprotic acid with initial concentration 'C', the concentration of H₃O⁺ is approximately equal to 'C'.
-
Weak Acids: These acids only partially dissociate in water. The equilibrium lies closer to the left, and a significant amount of the undissociated acid remains. Calculating the pH of a weak acid solution requires using the Ka value and often involves the quadratic formula or approximations.
Calculating the pH of a Monoprotic Acid Solution
The pH of a solution is a measure of its acidity, defined as the negative logarithm (base 10) of the hydronium ion concentration:
pH = -log₁₀[H₃O⁺]
Calculating the pH depends on whether the acid is strong or weak:
Calculating the pH of a Strong Monoprotic Acid Solution
For a strong monoprotic acid, the calculation is straightforward:
- Determine the concentration of H₃O⁺: Since the acid completely dissociates, the concentration of H₃O⁺ is equal to the initial concentration of the acid.
- Calculate the pH: Substitute the H₃O⁺ concentration into the pH equation.
Example: A 0.1 M solution of HCl will have a [H₃O⁺] of 0.1 M, resulting in a pH of -log₁₀(0.1) = 1.
Calculating the pH of a Weak Monoprotic Acid Solution
Calculating the pH of a weak monoprotic acid solution is more complex and often involves:
- Setting up an ICE table (Initial, Change, Equilibrium): This organizes the initial concentrations, the change in concentrations due to dissociation, and the equilibrium concentrations.
- Using the Ka expression: Substitute the equilibrium concentrations from the ICE table into the Ka expression.
- Solving for [H₃O⁺]: This often involves the quadratic formula or, if the acid is very weak (Ka is much smaller than the initial concentration), an approximation can be used, simplifying the calculation.
- Calculating the pH: Once [H₃O⁺] is found, the pH can be calculated using the pH equation.
Example: Consider a 0.1 M solution of acetic acid (Ka = 1.8 x 10⁻⁵). Using an ICE table and the Ka expression, we can solve for [H₃O⁺] and then calculate the pH. The approximation method is often suitable for weak acids, simplifying the calculation.
Factors Influencing the Strength of a Monoprotic Acid
Several factors influence the strength of a monoprotic acid:
- Bond Strength: Stronger bonds between the hydrogen atom and the rest of the molecule lead to weaker acids. The weaker the bond, the more readily the proton is released.
- Electronegativity: The electronegativity of the atom bonded to the hydrogen atom affects the acid's strength. Higher electronegativity leads to a more stable conjugate base, resulting in a stronger acid.
- Resonance Stabilization: If the conjugate base can be stabilized through resonance, it makes the acid stronger. Resonance delocalizes the negative charge, making the conjugate base more stable.
- Inductive Effects: Electron-withdrawing groups can stabilize the conjugate base through inductive effects, increasing the acid's strength.
Beyond the Basics: Titration and Buffer Solutions
The concepts discussed above form the foundation for understanding more advanced topics:
Acid-Base Titrations
Titration involves gradually adding a base to an acid solution (or vice-versa) to determine the concentration of the unknown solution. Titration curves for monoprotic acids have a distinct shape, with a sharp change in pH near the equivalence point (where the moles of acid and base are equal).
Buffer Solutions
Buffer solutions resist changes in pH upon the addition of small amounts of acid or base. They are often prepared by mixing a weak monoprotic acid and its conjugate base. The Henderson-Hasselbalch equation is used to calculate the pH of a buffer solution:
pH = pKa + log₁₀([A⁻] / [HA])
This equation shows the relationship between the pH, the pKa (negative logarithm of Ka), and the ratio of the concentrations of the conjugate base and the weak acid.
Applications of Monoprotic Acids
Monoprotic acids have widespread applications in various fields:
- Industrial Processes: Many industrial processes utilize strong monoprotic acids like HCl and HNO₃.
- Pharmaceuticals: Many pharmaceuticals are weak acids or bases. Understanding their acid-base properties is crucial for drug development and delivery.
- Food and Beverage Industry: Acetic acid (vinegar) is a common food additive, showcasing the importance of weak monoprotic acids in everyday life.
- Analytical Chemistry: Acid-base titrations are essential techniques in analytical chemistry, leveraging the properties of monoprotic acids.
Conclusion
Understanding the behavior of monoprotic acids when dissolved in water is essential for various chemical applications. From calculating pH values to understanding titration curves and buffer solutions, the principles discussed in this article provide a strong foundation for further exploration in acid-base chemistry. The key takeaway is the importance of the acid dissociation constant (Ka) in determining the extent of dissociation and, consequently, the acidity of the solution. By mastering these fundamental concepts, you'll gain a deeper appreciation of the intricate world of acid-base chemistry. Further exploration into polyprotic acids and more complex equilibrium systems will build upon this foundation, unlocking a greater understanding of chemical reactions in solution.
Latest Posts
Latest Posts
-
How Does A Shortcut Link To Another File
Mar 17, 2025
-
Cash Flows From Financing Activities Do Not Include
Mar 17, 2025
-
A Positive Return On Investment For Education Happens When
Mar 17, 2025
-
What Is The Value Of I
Mar 17, 2025
-
The Accounts In The Ledger Of Monroe Entertainment Co
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Enough Of A Monoprotic Acid Is Dissolved In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
