Draw The Structure Of An Eight Carbon Alkene
Holbox
Mar 22, 2025 · 6 min read
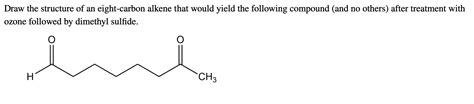
Table of Contents
- Draw The Structure Of An Eight Carbon Alkene
- Table of Contents
- Drawing the Structure of an Eight-Carbon Alkene: A Comprehensive Guide
- Understanding Isomerism in Octenes
- 1. Positional Isomerism: The Location of the Double Bond
- 2. Geometric (Cis-Trans) Isomerism: Double Bond Stereochemistry
- 3. Structural Isomerism: Branching of the Carbon Chain
- Drawing Octene Structures: Techniques and Considerations
- 1. Condensed Structural Formulas
- 2. Skeletal Formulas (Line-Angle Formulas)
- 3. Lewis Structures
- 4. 3D Representations
- Nomenclature of Octenes
- Applications and Importance of Understanding Octene Structures
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Drawing the Structure of an Eight-Carbon Alkene: A Comprehensive Guide
Alkenes, also known as olefins, are unsaturated hydrocarbons containing at least one carbon-carbon double bond. The general formula for an alkene is C<sub>n</sub>H<sub>2n</sub>, where 'n' represents the number of carbon atoms. This article will delve deep into the structural possibilities for an eight-carbon alkene (octene, C<sub>8</sub>H<sub>16</sub>), exploring isomerism, nomenclature, and practical considerations for drawing these structures. We will cover various drawing techniques and emphasize the importance of understanding structural differences for predicting chemical properties.
Understanding Isomerism in Octenes
The complexity of drawing an eight-carbon alkene stems from the significant number of isomers possible. Isomers are molecules with the same molecular formula but different structural arrangements. For octene (C<sub>8</sub>H<sub>16</sub>), we encounter several types of isomerism:
1. Positional Isomerism: The Location of the Double Bond
The double bond in an octene can be located between any two carbon atoms in the chain. This leads to a series of positional isomers. For example, 1-octene has the double bond between carbons 1 and 2, while 2-octene has it between carbons 2 and 3, and so on. There are seven possible positional isomers based solely on the position of the double bond:
- 1-Octene: CH<sub>2</sub>=CH-(CH<sub>2</sub>)<sub>6</sub>-CH<sub>3</sub>
- 2-Octene: CH<sub>3</sub>-CH=CH-(CH<sub>2</sub>)<sub>5</sub>-CH<sub>3</sub>
- 3-Octene: CH<sub>3</sub>-(CH<sub>2</sub>)-CH=CH-(CH<sub>2</sub>)<sub>4</sub>-CH<sub>3</sub>
- 4-Octene: CH<sub>3</sub>-(CH<sub>2</sub>)<sub>2</sub>-CH=CH-(CH<sub>2</sub>)<sub>3</sub>-CH<sub>3</sub>
- 5-Octene: CH<sub>3</sub>-(CH<sub>2</sub>)<sub>3</sub>-CH=CH-(CH<sub>2</sub>)<sub>2</sub>-CH<sub>3</sub>
- 6-Octene: CH<sub>3</sub>-(CH<sub>2</sub>)<sub>4</sub>-CH=CH-CH<sub>2</sub>-CH<sub>3</sub>
- 7-Octene: CH<sub>3</sub>-(CH<sub>2</sub>)<sub>5</sub>-CH=CH<sub>2</sub>
2. Geometric (Cis-Trans) Isomerism: Double Bond Stereochemistry
The carbon-carbon double bond exhibits restricted rotation. This leads to geometric isomerism, also known as cis-trans isomerism or E/Z isomerism. For each positional isomer (except 1-octene and 7-octene), two geometric isomers are possible:
- Cis (Z) isomer: The two highest priority substituents on each carbon of the double bond are on the same side of the double bond.
- Trans (E) isomer: The two highest priority substituents on each carbon of the double bond are on opposite sides.
For example, 2-octene has a cis-2-octene and a trans-2-octene isomer. This significantly increases the number of possible octene structures.
3. Structural Isomerism: Branching of the Carbon Chain
Beyond positional and geometric isomerism, octene can also exist as structural isomers with branched carbon chains. These isomers have the same molecular formula but differ in the arrangement of carbon atoms. The branching can lead to many more isomers, adding significant complexity to the structural possibilities. For instance, you could have a methyl group branching off, leading to isomers like 4-methyl-1-heptene or 2-methyl-1-heptene, and so on. The complexity increases exponentially with the number of carbons and the degree of branching.
Drawing Octene Structures: Techniques and Considerations
Several methods can be used to draw the structures of octenes:
1. Condensed Structural Formulas
This method represents the carbon backbone and attached hydrogens in a more compact form. For example, 1-octene is represented as CH<sub>2</sub>=CH(CH<sub>2</sub>)<sub>6</sub>CH<sub>3</sub>. While efficient in space, it lacks the visual clarity of other methods.
2. Skeletal Formulas (Line-Angle Formulas)
This method is particularly useful for depicting the carbon skeleton efficiently. Carbon atoms are represented by the vertices (corners and ends) of lines, and hydrogen atoms are implied. Each vertex represents a carbon atom, and sufficient hydrogens are added to satisfy carbon's tetravalency (four bonds). Double bonds are explicitly shown with two lines. This method is excellent for visualizing the overall structure and identifying branching points.
3. Lewis Structures
Lewis structures explicitly show all atoms and bonds, including lone pairs of electrons. While more detailed, they can be cumbersome for larger molecules like octenes, especially when depicting multiple isomers.
4. 3D Representations
For a deeper understanding of geometric isomerism, 3D representations using wedges and dashes are essential. Wedges represent bonds projecting out of the plane of the paper (towards the viewer), while dashes represent bonds projecting behind the plane (away from the viewer). Solid lines indicate bonds in the plane of the paper. This method is crucial for visualizing the spatial arrangement of atoms and understanding cis-trans differences.
Nomenclature of Octenes
The IUPAC (International Union of Pure and Applied Chemistry) nomenclature provides a systematic way to name alkenes. The rules include:
- Identify the longest continuous carbon chain containing the double bond. This chain forms the parent alkene name (octene in this case).
- Number the carbon atoms in the chain, starting from the end closest to the double bond.
- Indicate the position of the double bond using the lower number of the two carbons involved in the double bond.
- Indicate the position and name of any substituents (branches) on the chain.
- Specify the stereochemistry (cis/trans or E/Z) if relevant.
For example, (Z)-5-methyl-2-hexene indicates a hexene (6-carbon chain) with a methyl substituent on carbon 5, a double bond between carbons 2 and 3, and the methyl group and the longest chain are on the same side of the double bond.
Applications and Importance of Understanding Octene Structures
The ability to draw and understand the structures of octenes is crucial in various fields, including:
- Organic Chemistry: Understanding isomerism is vital for predicting and explaining the reactivity of alkenes. Different isomers will have different physical and chemical properties (e.g., boiling points, reactivity towards electrophiles).
- Polymer Chemistry: Octenes are monomers used in the production of various polymers, with different isomers leading to polymers with distinct properties. The structure of the monomer significantly influences the properties of the resulting polymer.
- Petroleum Industry: Octenes are components of petroleum and its various fractions. Understanding their structure is important for refining and processing petroleum products.
- Biochemistry: Some octene derivatives or isomers may have biological significance, requiring structural analysis for understanding their function.
Conclusion
Drawing the structure of an eight-carbon alkene is not a simple task due to the considerable number of isomers possible. This complexity arises from various types of isomerism, including positional, geometric, and structural isomerism. Mastering different drawing techniques, understanding IUPAC nomenclature, and appreciating the implications of structural variations are crucial for anyone working in fields related to organic chemistry, polymers, or the petroleum industry. A thorough grasp of these concepts is essential for predicting and interpreting the chemical and physical properties of these important compounds. The examples provided throughout this article serve as a foundation for exploring the many possibilities and nuances associated with drawing and understanding the structures of octenes. Further investigation into specific isomers and reactions is encouraged to develop a complete understanding of alkene chemistry.
Latest Posts
Latest Posts
-
Prepare The Current Year End Balance Sheet For Armani Company
Mar 23, 2025
-
Based On The Sign Of The Standard Cell Potential
Mar 23, 2025
-
Quad Enterprises Is Considering A New Three Year
Mar 23, 2025
-
Choose The Statement Below That Explains What Closing Means
Mar 23, 2025
-
Draw The Shear And Moment Diagrams For The Beam
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Draw The Structure Of An Eight Carbon Alkene . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
