Based On The Sign Of The Standard Cell Potential
Holbox
Mar 23, 2025 · 6 min read
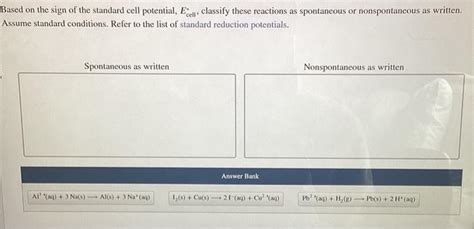
Table of Contents
- Based On The Sign Of The Standard Cell Potential
- Table of Contents
- Based on the Sign of the Standard Cell Potential: Understanding Electrochemical Reactions
- Understanding Standard Cell Potential (E°cell)
- The Significance of the Sign of E°cell
- Positive E°cell: Spontaneous Reactions
- Negative E°cell: Non-Spontaneous Reactions
- Zero E°cell: Equilibrium
- Relationship between E°cell and the Equilibrium Constant (K)
- Factors Affecting Standard Cell Potential
- Applications of Standard Cell Potential
- Beyond Standard Conditions: The Nernst Equation
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Based on the Sign of the Standard Cell Potential: Understanding Electrochemical Reactions
The standard cell potential, denoted as E°cell, is a crucial parameter in electrochemistry that provides valuable insights into the spontaneity and feasibility of electrochemical reactions. This article delves deep into the significance of the sign of E°cell, exploring its implications for reaction direction, equilibrium constant, and practical applications. We'll cover various aspects, ensuring a comprehensive understanding of this fundamental concept.
Understanding Standard Cell Potential (E°cell)
The standard cell potential is the potential difference between two half-cells under standard conditions: 298 K (25°C), 1 atm pressure, and 1 M concentration of all ions involved. It's a measure of the driving force of a redox reaction, essentially indicating the tendency of the reaction to occur spontaneously. This potential is calculated by summing the standard reduction potentials (E°red) of the half-reactions involved:
E°cell = E°red (cathode) – E°red (anode)
Here, the cathode is where reduction occurs (gain of electrons), and the anode is where oxidation occurs (loss of electrons). Standard reduction potentials are tabulated for various half-reactions, allowing for straightforward calculation of the standard cell potential for countless electrochemical cells.
The Significance of the Sign of E°cell
The sign of E°cell holds paramount importance in predicting the spontaneity and direction of a redox reaction. It directly correlates with the Gibbs Free Energy change (ΔG°) of the reaction:
ΔG° = -nFE°cell
where:
- n is the number of moles of electrons transferred in the balanced redox reaction.
- F is Faraday's constant (96,485 C/mol).
Positive E°cell: Spontaneous Reactions
A positive E°cell indicates that the redox reaction is spontaneous under standard conditions. This means the reaction will proceed in the forward direction without external intervention. The Gibbs Free Energy change (ΔG°) will be negative, further confirming spontaneity. In such cases, the cell will produce a voltage, acting as a galvanic cell or battery. Electrons will flow spontaneously from the anode (oxidation) to the cathode (reduction).
Example: A classic example is the Daniell cell, composed of a zinc anode and a copper cathode. The positive E°cell ensures that zinc readily oxidizes, releasing electrons that flow through the external circuit to reduce copper ions.
Negative E°cell: Non-Spontaneous Reactions
A negative E°cell signifies that the redox reaction is non-spontaneous under standard conditions. This means the reaction will not proceed in the forward direction without the input of external energy. The Gibbs Free Energy change (ΔG°) will be positive. To drive such a reaction, external energy must be supplied, turning the cell into an electrolytic cell. The reaction will proceed only when an external voltage greater than the absolute value of E°cell is applied. Electrons are forced to flow from the cathode to the anode, effectively reversing the spontaneous reaction.
Example: The electrolysis of water is a prime example. Under standard conditions, the decomposition of water into hydrogen and oxygen has a negative E°cell, requiring an external voltage to drive the reaction.
Zero E°cell: Equilibrium
A zero E°cell indicates that the redox reaction is at equilibrium under standard conditions. There is no net driving force in either direction. The Gibbs Free Energy change (ΔG°) will be zero. This signifies that the forward and reverse reaction rates are equal.
Relationship between E°cell and the Equilibrium Constant (K)
The standard cell potential is directly related to the equilibrium constant (K) of the redox reaction through the Nernst equation:
E°cell = (RT/nF)lnK
At standard temperature (298 K), this simplifies to:
E°cell = (0.0592/n)logK
This equation highlights the powerful connection between thermodynamics (E°cell and ΔG°) and kinetics (K). A positive E°cell translates to a K value greater than 1, indicating that the equilibrium favors the products. Conversely, a negative E°cell results in a K value less than 1, suggesting that the equilibrium favors the reactants.
Factors Affecting Standard Cell Potential
Several factors can influence the standard cell potential, although the standard conditions aim to minimize these variations:
-
Temperature: While standard conditions dictate 298 K, changes in temperature affect the equilibrium constant and consequently, the cell potential. The Nernst equation incorporates temperature dependence.
-
Concentration: Deviation from 1 M concentration of ions alters the cell potential. The Nernst equation accounts for this by incorporating the actual ion concentrations.
-
Pressure: Gas pressures deviating from 1 atm will influence the cell potential, particularly in reactions involving gaseous reactants or products.
-
Nature of Electrodes: The choice of electrodes significantly impacts the reduction potentials and hence the overall cell potential.
Applications of Standard Cell Potential
Understanding the sign of the standard cell potential is crucial in various applications:
-
Battery Design: Designing efficient batteries requires selecting redox couples with sufficiently positive E°cell to deliver the desired voltage and energy density.
-
Corrosion Prediction: Analyzing E°cell values helps predict the susceptibility of metals to corrosion. Metals with more negative reduction potentials are more prone to oxidation (corrosion).
-
Electroplating: Controlling the cell potential is essential for effective electroplating, ensuring the desired deposition rate and quality.
-
Electrolysis: Determining the minimum voltage required for electrolysis relies on the negative E°cell of the desired reaction.
-
Sensors and Detectors: Electrochemical sensors exploit the potential changes arising from specific redox reactions to detect and quantify various analytes.
Beyond Standard Conditions: The Nernst Equation
While E°cell provides a valuable indication under standard conditions, real-world electrochemical systems rarely operate under these ideal circumstances. The Nernst equation is crucial in calculating the cell potential under non-standard conditions:
Ecell = E°cell – (RT/nF)lnQ
where Q is the reaction quotient, a ratio of the activities (approximated by concentrations) of products to reactants at a given moment. This equation allows for accurate predictions of cell potential under varied concentrations, pressures, and temperatures.
Conclusion
The sign of the standard cell potential is a powerful tool for understanding and predicting the spontaneity and feasibility of electrochemical reactions. Its close relationship with Gibbs Free Energy and the equilibrium constant allows for a comprehensive thermodynamic and kinetic analysis. Moreover, its applications in diverse fields highlight its importance in both theoretical understanding and practical applications. While standard conditions provide a valuable starting point, the Nernst equation allows for more accurate predictions under real-world, non-standard conditions. A thorough grasp of the sign of E°cell and its implications is indispensable for anyone working in electrochemistry or related fields. Mastering this concept lays the foundation for a deeper understanding of the intricate world of redox reactions and their applications in various technologies.
Latest Posts
Latest Posts
-
Match The Hormone Abbreviations With Their Function
Mar 26, 2025
-
Deserving Of Large Investment To Support Continued Growth
Mar 26, 2025
-
A Disadvantage Of Is That It
Mar 26, 2025
-
Which Of The Following Is Not A Manufacturing Cost Category
Mar 26, 2025
-
Pharmacotherapeutics For Advanced Practice A Practical Approach
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Based On The Sign Of The Standard Cell Potential . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
