Draw The Structural Formula Of Diethylacetylene
Holbox
Mar 19, 2025 · 5 min read
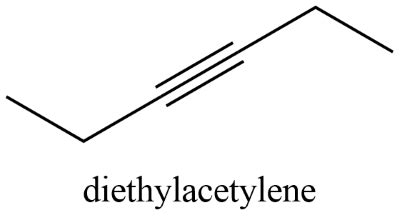
Table of Contents
Drawing the Structural Formula of Diethylacetylene: A Comprehensive Guide
Diethylacetylene, also known as 3-hexyne, is a simple alkyne with a fascinating structural formula. Understanding its structure is key to understanding its chemical properties and reactivity. This comprehensive guide will delve into the detailed drawing of its structural formula, exploring different representation methods and clarifying common misconceptions. We’ll also touch upon its nomenclature and some of its key characteristics.
Understanding Alkyne Structure
Before diving into diethylacetylene specifically, let's establish a foundation in alkyne chemistry. Alkynes are hydrocarbons containing at least one carbon-carbon triple bond. This triple bond consists of one sigma (σ) bond and two pi (π) bonds, resulting in a linear geometry around the triple-bonded carbons. This linear geometry significantly impacts the molecule's overall shape and reactivity.
Key Features of Alkynes:
- Triple Bond: The defining feature, contributing to its higher reactivity compared to alkanes and alkenes.
- Linear Geometry: The atoms directly attached to the triple-bonded carbons are arranged linearly (180° bond angle).
- Unsaturation: Alkynes are unsaturated hydrocarbons, meaning they contain fewer hydrogen atoms than the maximum possible for a given number of carbon atoms.
- Acidity: Terminal alkynes (those with a triple bond at the end of the chain) exhibit a degree of acidity due to the sp hybridization of the terminal carbon.
Drawing the Structural Formula of Diethylacetylene (3-Hexyne)
Diethylacetylene, systematically named 3-hexyne, has six carbon atoms arranged in a chain with a triple bond between the third and fourth carbons. Let's explore various ways to represent its structural formula:
1. Condensed Structural Formula
This representation shows all atoms but omits explicit bonds, implying connections based on chemical rules. For diethylacetylene:
CH₃CH₂C≡CCH₂CH₃
This is a concise way to show the connectivity of the atoms.
2. Expanded Structural Formula
This method shows all atoms and all bonds explicitly. This offers a clearer visual representation of the molecule's structure.
CH₃ CH₃
| |
CH₂ CH₂
| |
C≡C
| |
H−C≡C−H
Notice the linear geometry around the triple bond. The carbons involved in the triple bond only have two other atoms attached; hence their linear structure.
3. Skeletal (Line-Angle) Formula
This is a simplified representation commonly used in organic chemistry. Carbon atoms are implied at the intersections and ends of lines. Hydrogen atoms attached to carbon are generally omitted for clarity. The triple bond is represented by three lines.
CH₃CH₂-C≡C-CH₂CH₃
This becomes:
CH₃CH₂ CH₂CH₃
││
≡
This is the most compact and efficient way to depict the structure.
4. 3D Representation
While not a standard "formula", a three-dimensional representation helps visualize the molecule's shape. Due to the linear geometry around the triple bond, the molecule is not entirely linear but has a slightly bent shape due to the arrangement of the ethyl groups. Software packages can generate accurate 3D models, providing a more intuitive understanding of the molecule's spatial arrangement. Consider the "zig-zag" conformation commonly depicted in organic chemistry; this is a simplification of the actual 3D structure, which will be more flexible and potentially adopt various conformations in solution or the gas phase.
Nomenclature of Diethylacetylene (3-Hexyne)
The systematic name, 3-hexyne, is derived from IUPAC (International Union of Pure and Applied Chemistry) nomenclature rules. Let's break down the name:
- Hex: Indicates the presence of six carbon atoms in the main chain.
- yne: Denotes the presence of a triple bond (alkyne).
- 3: Specifies the position of the triple bond, starting the numbering from the end closest to the triple bond. It is located between the third and fourth carbon atoms.
The common name, diethylacetylene, reflects the molecule's structure as two ethyl groups (CH₂CH₃) attached to an acetylene group (C≡C).
Chemical Properties and Reactivity of Diethylacetylene
The presence of the triple bond dictates much of diethylacetylene's reactivity. It's more reactive than alkenes due to the presence of two pi bonds. Key reactions include:
- Hydrogenation: Addition of hydrogen across the triple bond, potentially in two steps to form first an alkene and then an alkane.
- Halogenation: Addition of halogens (e.g., chlorine, bromine) across the triple bond.
- Hydrohalogenation: Addition of hydrogen halides (e.g., HCl, HBr) across the triple bond, often following Markovnikov's rule.
- Hydration: Addition of water across the triple bond, forming a ketone.
Applications and Importance of Diethylacetylene
Although not as prevalent as other alkynes, diethylacetylene finds niche applications in various chemical processes. It serves as a valuable intermediate in the synthesis of more complex organic molecules, particularly in the pharmaceutical and materials science industries. Its reactivity makes it a suitable building block for creating diverse chemical structures. Due to its relatively simple structure, it serves as a good model compound for studying alkyne reactivity and properties.
Common Misconceptions about Diethylacetylene's Structure
A common mistake is misplacing the triple bond. Remember that the numbering should be done such that the triple bond gets the lowest possible number. The correct placement is between carbons 3 and 4, not between other carbon pairs. Also, be mindful of representing the linear geometry around the triple bond accurately. Failure to consider this linear geometry can lead to inaccurate depictions of the molecule's three-dimensional structure.
Conclusion
Drawing the structural formula of diethylacetylene, while seemingly straightforward, requires a good understanding of alkyne chemistry and proper application of chemical nomenclature. Mastering the different representation methods – condensed, expanded, skeletal, and 3D – is crucial for effective communication in organic chemistry. Understanding its structure provides insight into its chemical properties and potential applications. By applying the principles discussed in this comprehensive guide, you can confidently draw and interpret the structure of diethylacetylene and other similar organic molecules. Remember to practice and utilize various representation methods to enhance your understanding and skills in structural organic chemistry.
Latest Posts
Latest Posts
-
Which Of The Following Statements Best Describes Microtubules
Mar 19, 2025
-
In A Windows Environment How Many Hops To Reach Google Com
Mar 19, 2025
-
A Flexible Budget May Be Prepared
Mar 19, 2025
-
Be All You Can Be Is An Example Of A
Mar 19, 2025
-
What Is The Particular Significance Of Valence Electrons
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Draw The Structural Formula Of Diethylacetylene . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
