Draw The Product Of The Reaction Shown Below
Holbox
Mar 18, 2025 · 5 min read
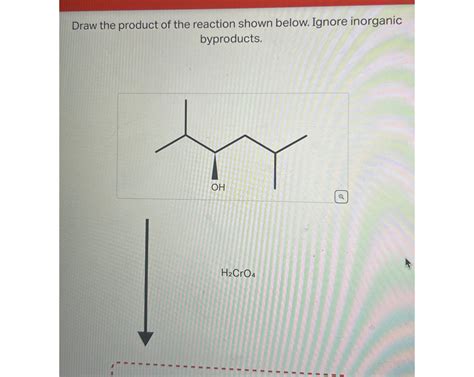
Table of Contents
Drawing the Product of Organic Reactions: A Comprehensive Guide
Predicting the product of an organic reaction is a fundamental skill for any organic chemist. This skill requires a deep understanding of reaction mechanisms, functional group transformations, and stereochemical considerations. This article will delve into the process of predicting reaction products, focusing on common reaction types and providing a step-by-step approach to accurately drawing the product of a given reaction. We will explore several examples to illustrate the concepts, addressing complexities such as regioselectivity and stereoselectivity. Understanding these aspects will significantly improve your ability to predict reaction outcomes.
Understanding Reaction Mechanisms
Before predicting the product, it's crucial to understand the underlying reaction mechanism. The mechanism dictates the pathway of electron flow, determining which bonds are broken and formed, and consequently, the final product. Common reaction mechanisms include:
-
SN1 (Substitution Nucleophilic Unimolecular): This mechanism involves a carbocation intermediate, leading to racemization at the reaction center. It favors tertiary substrates and polar protic solvents.
-
SN2 (Substitution Nucleophilic Bimolecular): This mechanism is a concerted reaction, proceeding through a transition state. It favors primary substrates and polar aprotic solvents. It also leads to inversion of configuration at the reaction center.
-
E1 (Elimination Unimolecular): This mechanism proceeds through a carbocation intermediate, often competing with SN1. It favors tertiary substrates and polar protic solvents. It leads to the formation of alkenes.
-
E2 (Elimination Bimolecular): This mechanism is a concerted reaction, requiring a strong base. It leads to the formation of alkenes and often shows regioselectivity (Zaitsev's rule).
-
Addition Reactions: These reactions involve the addition of a reagent across a double or triple bond. Examples include electrophilic addition to alkenes and nucleophilic addition to carbonyl compounds.
Step-by-Step Approach to Predicting Reaction Products
To accurately predict the product of a reaction, follow these steps:
-
Identify the Functional Groups: Carefully identify all functional groups present in the reactants. This will help determine the likely reaction type.
-
Determine the Reaction Type: Based on the functional groups and the reagents used, determine the type of reaction occurring (SN1, SN2, E1, E2, addition, etc.). Consider the reaction conditions (solvent, temperature, presence of a catalyst).
-
Draw the Mechanism: Draw a detailed mechanism showing the movement of electrons. This helps visualize bond breaking and formation. Pay attention to any intermediates formed.
-
Identify the Major Product: Based on the mechanism, identify the major product formed. Consider regioselectivity and stereoselectivity, which are often dictated by steric hindrance and electronic effects.
-
Consider Side Reactions: Some reactions may have competing pathways, leading to side products. Consider the possibility of side reactions and their relative yields.
-
Verify Stereochemistry: For reactions involving chiral centers, carefully consider the stereochemistry of the product. Will the reaction lead to inversion, retention, or racemization of configuration?
-
Draw the Final Product: Finally, draw the structure of the major product, including all stereochemical details.
Examples and Detailed Explanations
Let's illustrate this process with some examples:
Example 1: SN2 Reaction
(Reactants): CH3CH2Br + NaCN (in DMF)
-
Functional Groups: Alkyl halide (CH3CH2Br), cyanide ion (CN⁻).
-
Reaction Type: SN2 reaction (due to primary alkyl halide, strong nucleophile, and aprotic solvent).
-
Mechanism: The cyanide ion attacks the carbon atom bearing the bromine atom from the backside, leading to inversion of configuration. The bromide ion leaves.
-
Major Product: CH3CH2CN
-
Side Reactions: Minimal side reactions are expected under these conditions.
-
Stereochemistry: Inversion of configuration occurs at the carbon atom.
-
Final Product: CH3CH2CN (No stereochemistry to denote in this case)
Example 2: E1 Reaction
(Reactants): (CH3)3COH + H2SO4 (heat)
-
Functional Groups: Tertiary alcohol, sulfuric acid.
-
Reaction Type: E1 elimination (due to tertiary alcohol and acidic conditions).
-
Mechanism: Protonation of the alcohol forms a good leaving group (water). Loss of water forms a tertiary carbocation. A proton is abstracted from a beta-carbon to form the alkene.
-
Major Product: (CH3)2C=CH2 (Zaitsev's rule favors the more substituted alkene).
-
Side Reactions: Some SN1 product might form, but E1 will be dominant.
-
Stereochemistry: The alkene product will be a mixture of isomers (E and Z isomers possible) due to the planar nature of the carbocation intermediate.
-
Final Product: (CH3)2C=CH2 (Show a mixture of E and Z isomers if appropriate).
Example 3: Electrophilic Addition
(Reactants): CH2=CH2 + Br2
-
Functional Groups: Alkene, bromine.
-
Reaction Type: Electrophilic addition.
-
Mechanism: Bromine adds across the double bond via a bromonium ion intermediate. Attack by bromide ion leads to the product.
-
Major Product: BrCH2CH2Br (1,2-dibromoethane)
-
Side Reactions: None significant.
-
Stereochemistry: Anti-addition occurs (bromines add to opposite sides of the double bond).
-
Final Product: BrCH2CH2Br
Example 4: Grignard Reaction
(Reactants): CH3MgBr + CH3CHO
-
Functional Groups: Grignard reagent (organomagnesium halide), aldehyde.
-
Reaction Type: Nucleophilic addition.
-
Mechanism: The Grignard reagent acts as a nucleophile, attacking the carbonyl carbon. Protonation gives the alcohol.
-
Major Product: CH3CH(OH)CH3 (propan-2-ol)
-
Side Reactions: Can be affected by moisture and other reactive species.
-
Stereochemistry: The reaction creates a new chiral center, leading to a racemic mixture.
-
Final Product: CH3CH(OH)CH3 (indicate racemic mixture).
Advanced Considerations: Regioselectivity and Stereoselectivity
-
Regioselectivity: This refers to the preferential formation of one constitutional isomer over another. Zaitsev's rule predicts the more substituted alkene will be the major product in elimination reactions. Markovnikov's rule predicts the addition of a proton to the more substituted carbon in electrophilic addition to alkenes.
-
Stereoselectivity: This refers to the preferential formation of one stereoisomer over another. SN2 reactions lead to inversion of configuration. Electrophilic addition to alkenes can lead to syn or anti addition depending on the mechanism. E1 reactions often lead to a mixture of stereoisomers due to carbocation intermediates.
By carefully analyzing the reaction type, mechanisms, and stereochemical considerations, you can accurately predict the products of organic reactions. Practice is key to mastering this skill. Work through numerous examples and progressively challenge yourself with more complex reactions to improve your understanding. Remember to always check your work and ensure the product is consistent with the reaction mechanism and the rules of regioselectivity and stereoselectivity.
Latest Posts
Latest Posts
-
What Is The Product Of This Reaction
Mar 18, 2025
-
Record The Entry To Close The Dividends Account
Mar 18, 2025
-
Which Of The Following Best Describes The Operational Period Briefing
Mar 18, 2025
-
The Second Largest Number Of Pacs Are Those Associated With
Mar 18, 2025
-
The Accompanying Graph Represents Haydens Fro Yo
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Draw The Product Of The Reaction Shown Below . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
