Draw The Major Organic Product Of This Reaction
Holbox
Mar 28, 2025 · 5 min read
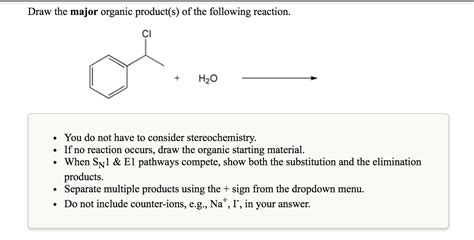
Table of Contents
- Draw The Major Organic Product Of This Reaction
- Table of Contents
- Drawing the Major Organic Product: A Comprehensive Guide to Reaction Prediction
- Understanding Reaction Mechanisms: The Foundation of Prediction
- Factors Influencing Product Formation: Regioselectivity and Stereoselectivity
- Worked Examples: Predicting Major Organic Products
- Advanced Considerations: Competing Reactions and Kinetic vs. Thermodynamic Control
- Conclusion: Mastering Organic Product Prediction
- Latest Posts
- Latest Posts
- Related Post
Drawing the Major Organic Product: A Comprehensive Guide to Reaction Prediction
Predicting the major organic product of a reaction is a fundamental skill in organic chemistry. It requires a deep understanding of reaction mechanisms, functional group transformations, and the principles of regio- and stereoselectivity. This comprehensive guide will equip you with the tools to confidently draw the major organic product for a wide range of reactions. We'll explore various reaction types, discuss crucial considerations for predicting outcomes, and provide worked examples to solidify your understanding.
Understanding Reaction Mechanisms: The Foundation of Prediction
Before diving into specific reactions, it's crucial to grasp the underlying reaction mechanism. The mechanism dictates the step-by-step process of bond breaking and bond formation, ultimately determining the structure of the product. Common mechanisms include:
-
SN1 (Substitution Nucleophilic Unimolecular): This reaction involves a two-step process: the formation of a carbocation intermediate followed by nucleophilic attack. The stability of the carbocation intermediate is paramount in determining the major product. More substituted carbocations (tertiary > secondary > primary) are more stable due to hyperconjugation.
-
SN2 (Substitution Nucleophilic Bimolecular): This reaction is a concerted process, meaning bond breaking and bond formation occur simultaneously. Stereochemistry is crucial in SN2 reactions. The nucleophile attacks the substrate from the backside, leading to inversion of configuration at the stereocenter. Steric hindrance significantly impacts the reaction rate.
-
E1 (Elimination Unimolecular): Similar to SN1, E1 reactions involve a carbocation intermediate. The base then abstracts a proton from a carbon adjacent to the carbocation, leading to the formation of a double bond. Zaitsev's rule typically governs the major product, predicting the formation of the more substituted alkene.
-
E2 (Elimination Bimolecular): This concerted reaction involves the simultaneous abstraction of a proton by a base and departure of a leaving group. Stereochemistry is critical; anti-periplanar arrangement of the proton and leaving group is favored. Zaitsev's rule also applies to E2 reactions.
-
Addition Reactions: These reactions involve the addition of a reagent across a multiple bond (double or triple). Markovnikov's rule often applies to the addition of unsymmetrical reagents to alkenes, predicting that the more substituted carbon will receive the more electronegative atom.
Factors Influencing Product Formation: Regioselectivity and Stereoselectivity
Several factors influence which product is formed preferentially:
-
Regioselectivity: This refers to the preferential formation of one regioisomer over another. Markovnikov's rule and Zaitsev's rule are examples of regioselectivity principles. The electronic and steric effects of substituents play a vital role.
-
Stereoselectivity: This refers to the preferential formation of one stereoisomer (e.g., enantiomer or diastereomer) over another. The stereochemistry of the starting material, the reaction mechanism, and the steric effects of reagents significantly influence stereoselectivity.
-
Steric Hindrance: Bulky substituents can hinder reaction pathways, favoring less hindered products.
-
Carbocation Stability: In reactions involving carbocation intermediates, the stability of the intermediate dictates the product distribution.
-
Leaving Group Ability: Good leaving groups (e.g., halides, tosylates) readily depart, facilitating the reaction.
Worked Examples: Predicting Major Organic Products
Let's apply these concepts to specific reaction examples.
Example 1: SN1 Reaction
Consider the reaction of tert-butyl bromide with methanol. This is a classic SN1 reaction.
(CH3)3CBr + CH3OH → ?
The tert-butyl cation is formed as an intermediate. Methanol, acting as a nucleophile, attacks the carbocation, forming tert-butyl methyl ether as the major product. The reaction proceeds with racemization because the carbocation is planar.
Example 2: SN2 Reaction
Consider the reaction of bromomethane with sodium hydroxide.
CH3Br + NaOH → ?
This is an SN2 reaction. The hydroxide ion attacks the carbon atom bonded to the bromine from the backside, resulting in inversion of configuration. The major product is methanol.
Example 3: E1 Reaction
Consider the dehydration of 2-methyl-2-butanol with sulfuric acid.
(CH3)2CHCH(OH)CH3 + H2SO4 → ?
This is an E1 reaction. A carbocation intermediate is formed, followed by proton abstraction to form an alkene. Zaitsev's rule predicts that the more substituted alkene (2-methyl-2-butene) will be the major product.
Example 4: E2 Reaction
Consider the reaction of 2-bromobutane with potassium tert-butoxide.
CH3CHBrCH2CH3 + (CH3)3COK → ?
This is an E2 reaction. The bulky tert-butoxide base preferentially abstracts a proton from the less hindered β-carbon, leading to the formation of 2-butene as the major product (Hofmann product, in contrast to Zaitsev's rule). The anti-periplanar geometry is crucial for this reaction.
Example 5: Addition Reaction (Markovnikov's Rule)
Consider the addition of HBr to propene.
CH3CH=CH2 + HBr → ?
This is an electrophilic addition reaction. The hydrogen atom adds to the less substituted carbon (Markovnikov's rule), resulting in 2-bromopropane as the major product.
Advanced Considerations: Competing Reactions and Kinetic vs. Thermodynamic Control
Many reactions can proceed through multiple pathways, leading to the formation of several products. Understanding competing reactions is critical for predicting the major product. For instance, a substrate might undergo both SN1 and E1 reactions depending on the reaction conditions. Kinetic control favors the product formed faster, while thermodynamic control favors the more stable product. Factors like temperature, solvent, and concentration can influence whether a reaction is under kinetic or thermodynamic control.
Conclusion: Mastering Organic Product Prediction
Predicting the major organic product of a reaction is a skill honed through practice and a thorough understanding of reaction mechanisms and the principles of regio- and stereoselectivity. By mastering these concepts and considering factors such as carbocation stability, steric hindrance, and competing pathways, you can significantly enhance your ability to accurately predict reaction outcomes and advance your understanding of organic chemistry. Remember, practice is key! Work through numerous examples, and don't hesitate to consult textbooks and resources for further clarification. With consistent effort, you'll become proficient in drawing the major organic product for a wide range of reactions.
Latest Posts
Latest Posts
-
A Critical Analysis Based On Heuristics Will Lead To
Apr 01, 2025
-
Predict The Major Organic Product Of The Following Reaction
Apr 01, 2025
-
Label The Floors Of The Hotel
Apr 01, 2025
-
Which Of The Following Is Not A Possible R Value
Apr 01, 2025
-
Record The Adjusting Entry For Uncollectible Accounts
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Draw The Major Organic Product Of This Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
