Predict The Major Organic Product Of The Following Reaction
Holbox
Apr 01, 2025 · 5 min read
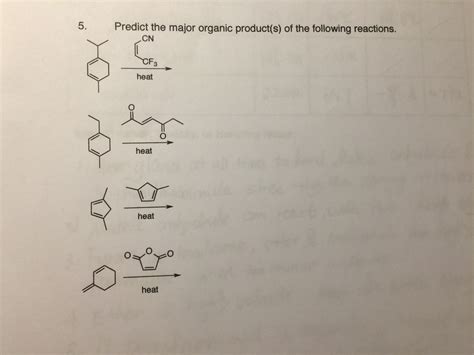
Table of Contents
- Predict The Major Organic Product Of The Following Reaction
- Table of Contents
- Predicting the Major Organic Product: A Comprehensive Guide
- Understanding Reaction Mechanisms: The Foundation of Prediction
- 1. Nucleophilic Substitution (SN1 & SN2)
- 2. Elimination Reactions (E1 & E2)
- 3. Addition Reactions
- 4. Oxidation and Reduction Reactions
- Factors Influencing Product Selectivity
- 1. Steric Hindrance
- 2. Carbocation Stability
- 3. Nucleophile/Base Strength and Sterics
- 4. Solvent Effects
- 5. Temperature
- Applying the Principles: Example Reactions and Predictions
- Advanced Considerations: Regioselectivity and Stereoselectivity
- Conclusion: A Systematic Approach to Prediction
- Latest Posts
- Latest Posts
- Related Post
Predicting the Major Organic Product: A Comprehensive Guide
Predicting the major organic product of a reaction is a fundamental skill in organic chemistry. It requires a deep understanding of reaction mechanisms, functional group transformations, and the factors influencing reaction selectivity. This article delves into the process, providing a systematic approach to accurately predict the outcome of various organic reactions. We'll explore several key concepts and apply them to example reactions, illustrating the thought process behind predicting the major product.
Understanding Reaction Mechanisms: The Foundation of Prediction
Before attempting to predict the major product, a thorough understanding of the reaction mechanism is paramount. The mechanism outlines the step-by-step process of bond breaking and bond formation, providing insights into the intermediate species and transition states involved. Different mechanisms lead to different products, even with the same starting materials. Key mechanistic concepts include:
1. Nucleophilic Substitution (SN1 & SN2)
- SN2: A concerted mechanism involving a backside attack by the nucleophile, leading to inversion of configuration at the chiral center. Steric hindrance significantly impacts the rate; bulky substrates react slower. Strong nucleophiles favor SN2 reactions.
- SN1: A two-step mechanism involving carbocation formation followed by nucleophilic attack. Carbocation stability (tertiary > secondary > primary) dictates the reaction rate. Weak nucleophiles and protic solvents favor SN1 reactions. Racemization often occurs due to planar carbocation intermediate.
2. Elimination Reactions (E1 & E2)
- E2: A concerted mechanism requiring a strong base and often leading to the more substituted (Zaitsev's rule) alkene product. Steric factors influence the regioselectivity.
- E1: A two-step mechanism involving carbocation formation followed by base-induced proton abstraction. Similar to SN1, carbocation stability dictates the rate and regioselectivity, favoring the more substituted alkene.
3. Addition Reactions
Addition reactions typically involve the addition of a reagent across a multiple bond (C=C, C≡C, C=O). The regioselectivity and stereoselectivity are influenced by factors like Markovnikov's rule (for electrophilic additions to alkenes) and the steric hindrance of the reactants.
4. Oxidation and Reduction Reactions
These reactions involve changes in oxidation states. Predicting products requires understanding the oxidizing or reducing agent's strength and selectivity. For example, oxidizing agents like KMnO₄ and CrO₃ can oxidize alcohols to ketones or carboxylic acids, while reducing agents like LiAlH₄ can reduce ketones to alcohols.
Factors Influencing Product Selectivity
Several factors beyond the basic mechanism influence which product predominates:
1. Steric Hindrance
Bulky groups can hinder the approach of reactants, influencing both reaction rate and selectivity. In SN2 reactions, bulky substrates react slower, while in E2 reactions, the less hindered β-hydrogen is preferentially abstracted.
2. Carbocation Stability
In SN1 and E1 reactions, the stability of the carbocation intermediate is crucial. Tertiary carbocations are the most stable, followed by secondary and then primary. This stability dictates the regioselectivity of the reaction.
3. Nucleophile/Base Strength and Sterics
Strong nucleophiles favor SN2 reactions, while weak nucleophiles favor SN1 reactions. Similarly, strong bases favor E2 reactions, while weaker bases may favor E1 reactions. The steric bulk of the nucleophile/base also affects its reactivity.
4. Solvent Effects
Protic solvents (like water and alcohols) stabilize carbocations and favor SN1 and E1 reactions. Aprotic solvents (like DMSO and DMF) favor SN2 reactions.
5. Temperature
Higher temperatures often favor elimination reactions over substitution reactions, as elimination reactions generally have higher activation energies.
Applying the Principles: Example Reactions and Predictions
Let's examine a few examples to illustrate the application of these principles:
Example 1: Reaction of 2-bromobutane with sodium ethoxide in ethanol.
This reaction involves a secondary alkyl halide and a strong base. Both SN2 and E2 mechanisms are possible. However, the strong base and relatively high temperature favor the E2 mechanism. The major product will be the more substituted alkene according to Zaitsev's rule: 2-butene (a mixture of cis and trans isomers).
Example 2: Reaction of tert-butyl bromide with methanol.
Tertiary alkyl halides readily undergo SN1 reactions due to the stability of the tertiary carbocation. In this case, the major product is tert-butyl methyl ether formed through SN1 mechanism. The methanol acts as both the nucleophile and the solvent, stabilizing the carbocation intermediate.
Example 3: Acid-catalyzed hydration of propene.
This reaction follows Markovnikov's rule. The proton adds to the less substituted carbon, forming a more stable carbocation, which is then attacked by water. The major product is 2-propanol.
Example 4: Oxidation of a secondary alcohol with chromic acid.
Chromic acid is a strong oxidizing agent. Oxidation of a secondary alcohol yields a ketone. For example, oxidation of 2-propanol with chromic acid produces acetone.
Example 5: Reduction of a ketone with sodium borohydride.
Sodium borohydride (NaBH₄) is a mild reducing agent. Reduction of a ketone yields a secondary alcohol. For example, reduction of acetone with NaBH₄ produces 2-propanol.
Advanced Considerations: Regioselectivity and Stereoselectivity
Predicting the major product often involves considering regioselectivity (which isomer is formed) and stereoselectivity (which stereoisomer is formed).
-
Regioselectivity: Markovnikov's rule guides the regioselectivity in electrophilic additions to alkenes. In elimination reactions, Zaitsev's rule predicts the formation of the more substituted alkene.
-
Stereoselectivity: SN2 reactions proceed with inversion of configuration. SN1 and E1 reactions often lead to a mixture of stereoisomers due to the planar carbocation intermediate. Certain reagents and conditions can favor specific stereoisomers (e.g., syn or anti addition).
Conclusion: A Systematic Approach to Prediction
Predicting the major organic product requires a methodical approach combining knowledge of reaction mechanisms, influencing factors, and selectivity principles. By carefully analyzing the reactants, conditions, and potential mechanisms, one can accurately predict the major product formed in a given reaction. This skill is crucial for designing and understanding organic syntheses and interpreting experimental results. Remember, practice is key. Working through numerous examples and applying the concepts discussed here will significantly improve your predictive ability. Consistent review and understanding of fundamental organic chemistry principles are essential to mastering this important aspect of the subject.
Latest Posts
Latest Posts
-
A Mathematical Sentence With An Equal Symbol Used
Apr 05, 2025
-
Which Of The Following Is Correct About Environmental Policy
Apr 05, 2025
-
The Entry For Manufacturing Overhead Cost Applied To Jobs
Apr 05, 2025
-
Suppose Banks Increase Excess Reserves By
Apr 05, 2025
-
You Want To Move The Image Around In A Slide
Apr 05, 2025
Related Post
Thank you for visiting our website which covers about Predict The Major Organic Product Of The Following Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
