Draw The Major Organic Product Of The Reaction Shown
Holbox
Mar 13, 2025 · 6 min read
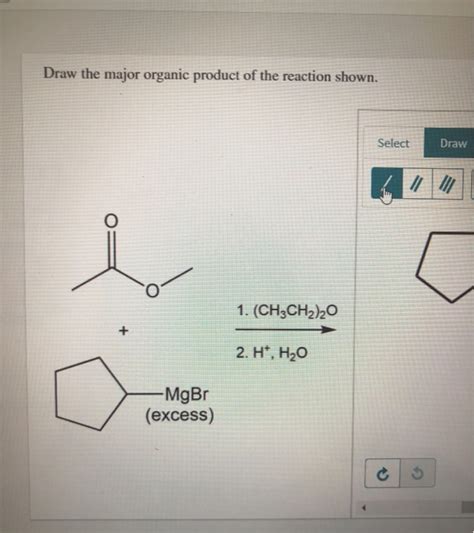
Table of Contents
Draw the Major Organic Product of the Reaction Shown: A Comprehensive Guide
Predicting the major organic product of a given reaction is a cornerstone of organic chemistry. This skill requires a deep understanding of reaction mechanisms, functional group transformations, and the factors that influence reaction selectivity. This comprehensive guide will delve into various reaction types, providing strategies and examples to help you accurately predict the major organic product. We'll explore the importance of understanding reaction mechanisms, steric hindrance, regioselectivity, and stereoselectivity in determining the outcome of organic reactions.
Understanding Reaction Mechanisms: The Key to Predicting Products
Before we jump into specific reactions, it's crucial to emphasize the importance of understanding the underlying reaction mechanism. A reaction mechanism is a detailed step-by-step description of how reactants transform into products. Knowing the mechanism allows us to predict not only the final product but also potential intermediates and side products.
Key mechanistic concepts include:
- Nucleophilic attack: A nucleophile (electron-rich species) attacks an electrophile (electron-deficient species).
- Electrophilic attack: An electrophile attacks a nucleophile.
- Proton transfer: Acid-base reactions involving the transfer of a proton.
- Rearrangements: Atoms or groups of atoms move within a molecule, often to form a more stable structure.
- Carbocation formation and stability: Understanding the stability of carbocations (positively charged carbon atoms) is crucial in predicting the major product in many reactions, especially those involving alkyl halides and alcohols. Tertiary carbocations are more stable than secondary, which are more stable than primary carbocations. This stability is due to the inductive effect and hyperconjugation.
Examples of Mechanistic Influence on Product Formation
Let's consider two common reactions to illustrate how the mechanism dictates the product:
1. SN1 vs. SN2 Reactions:
-
SN1 (Substitution Nucleophilic Unimolecular): This reaction proceeds through a carbocation intermediate. The rate-determining step is the formation of this carbocation. Therefore, the stability of the carbocation significantly influences the reaction outcome. SN1 reactions favor tertiary substrates and occur in polar protic solvents. Racemization often occurs at the chiral center.
-
SN2 (Substitution Nucleophilic Bimolecular): This reaction is a concerted mechanism, meaning the bond breaking and bond formation occur simultaneously. The nucleophile attacks the substrate from the backside, leading to inversion of configuration at the chiral center. SN2 reactions favor primary substrates and occur in polar aprotic solvents. Steric hindrance plays a significant role; bulky substrates react slower or not at all.
2. Electrophilic Aromatic Substitution:
This reaction involves the substitution of a hydrogen atom on an aromatic ring with an electrophile. The mechanism involves the formation of a resonance-stabilized carbocation intermediate (arenium ion). The position of the incoming electrophile depends on the directing effect of existing substituents on the aromatic ring. Electron-donating groups (e.g., -OH, -NH2) are ortho/para directing, while electron-withdrawing groups (e.g., -NO2, -COOH) are meta directing.
Regioselectivity and Stereoselectivity: Controlling Product Formation
Regioselectivity refers to the preferential formation of one regioisomer over others in a reaction. Stereoselectivity refers to the preferential formation of one stereoisomer over others. These concepts are crucial in predicting the major product, particularly in reactions involving multiple possible sites of reaction or the creation of chiral centers.
Factors influencing Regioselectivity:
- Steric hindrance: Bulky groups hinder the approach of reactants, favoring less hindered sites.
- Electronic effects: Electron-donating or withdrawing groups can influence the reactivity of different positions on a molecule.
- Resonance stabilization: Intermediates with greater resonance stabilization are favored.
Factors influencing Stereoselectivity:
- Stereospecific reactions: Reactions where the stereochemistry of the reactant dictates the stereochemistry of the product.
- Stereoselective reactions: Reactions that favor the formation of one stereoisomer over others, even if multiple stereoisomers are possible.
- Chirality and stereocenters: Understanding the number and configuration of chiral centers is essential in predicting the stereochemistry of products.
Predicting Products: A Step-by-Step Approach
Let's outline a step-by-step approach to predicting the major organic product of a reaction:
-
Identify the functional groups: Determine the functional groups present in the reactants and their reactivity.
-
Identify the type of reaction: Is it a substitution, addition, elimination, or rearrangement reaction?
-
Propose a mechanism: Draw out the mechanism, showing all intermediate steps. Pay close attention to carbocation stability, nucleophile/electrophile interactions, and stereochemistry.
-
Consider regioselectivity and stereoselectivity: Determine if the reaction will favor the formation of a specific regioisomer or stereoisomer.
-
Draw the major product: Based on your mechanism and consideration of regio- and stereoselectivity, draw the structure of the major organic product.
Examples of Predicting Major Organic Products
Let's work through a few examples to solidify these concepts. Remember to always consider the reaction mechanism, regioselectivity, and stereoselectivity.
Example 1: SN2 Reaction of 1-bromopropane with Sodium Methoxide
The reaction of 1-bromopropane with sodium methoxide (NaOCH3) in methanol is an SN2 reaction. The methoxide ion (OCH3-) acts as a nucleophile, attacking the carbon atom bonded to the bromine atom. This leads to inversion of configuration, resulting in the formation of methyl propyl ether.
Example 2: Acid-catalyzed dehydration of 2-methyl-2-butanol
The acid-catalyzed dehydration of 2-methyl-2-butanol is an elimination reaction. The reaction proceeds through a carbocation intermediate. The most stable carbocation is formed, leading to the major product: 2-methyl-2-butene. Zaitsev's rule predicts the formation of the more substituted alkene as the major product.
Example 3: Electrophilic Aromatic Substitution of Benzene with Bromine
The reaction of benzene with bromine in the presence of a Lewis acid catalyst (FeBr3) is an electrophilic aromatic substitution reaction. The bromine acts as the electrophile, attacking the benzene ring to form a bromobenzene. This reaction is not regiospecific since benzene is symmetrical.
Example 4: Addition of HBr to 1-butene
The addition of HBr to 1-butene follows Markovnikov's rule. The hydrogen atom adds to the carbon atom with more hydrogen atoms, and the bromine atom adds to the carbon atom with fewer hydrogen atoms. This results in the formation of 2-bromobutane.
Advanced Considerations: Beyond the Basics
While the concepts discussed above provide a strong foundation, several advanced considerations can further refine your ability to predict major organic products:
-
Kinetic vs. thermodynamic control: Some reactions can yield different products depending on the reaction conditions (temperature, time). Kinetic control favors the faster reaction, while thermodynamic control favors the more stable product.
-
Competition between different reaction pathways: A single reactant can undergo multiple reactions simultaneously. Understanding the relative rates of these reactions is crucial in predicting the major product.
-
Transition state theory: A more advanced approach involves analyzing the transition states of different reaction pathways to predict the most favorable pathway and consequently, the major product.
Conclusion: Mastering the Art of Predicting Organic Products
Predicting the major organic product of a reaction is a challenging but rewarding skill. By understanding reaction mechanisms, regioselectivity, stereoselectivity, and various factors influencing reaction pathways, you can confidently predict the outcome of a wide range of organic reactions. Practice is key—the more examples you work through, the more proficient you will become in this essential aspect of organic chemistry. Remember to approach each problem systematically, breaking it down into manageable steps, and always consult reliable resources to verify your predictions. This detailed guide will serve as a valuable reference point throughout your journey in mastering organic chemistry reaction prediction.
Latest Posts
Latest Posts
-
What Is Unique About Transduction Compared To Normal Bacteriophage Infection
Mar 13, 2025
-
The Basic Npv Investment Rule Is
Mar 13, 2025
-
The Most Fundemantal Criterion In Vendor Selection
Mar 13, 2025
-
Theo Needs To Enter A New Income Account Into Quickbooks
Mar 13, 2025
-
A Departmental Contribution To Overhead Report Is Based On
Mar 13, 2025
Related Post
Thank you for visiting our website which covers about Draw The Major Organic Product Of The Reaction Shown . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
