Draw The Correct Product For The Given Diels Alder Reaction
Holbox
Mar 15, 2025 · 6 min read
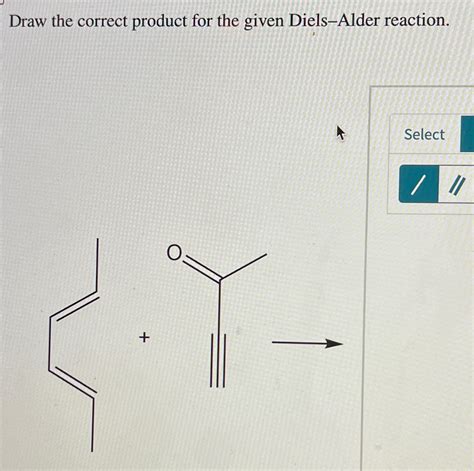
Table of Contents
Drawing the Correct Product for a Diels-Alder Reaction: A Comprehensive Guide
The Diels-Alder reaction is a cornerstone of organic chemistry, a powerful reaction forming six-membered rings. Understanding how to predict the product of a Diels-Alder reaction is crucial for any organic chemist. This comprehensive guide will walk you through the process, covering reaction mechanisms, regioselectivity, stereoselectivity, and common pitfalls to avoid. We'll explore how to correctly draw the product, including the stereochemistry, for various Diels-Alder reactions.
Understanding the Diels-Alder Reaction Mechanism
The Diels-Alder reaction is a /17%3A_Pericyclic_Reactions/17.02%3A_The_Diels-Alder_Reaction), meaning it occurs in a single step without any intermediates. A conjugated diene (containing four π electrons) reacts with a dienophile (containing two π electrons) to form a cyclohexene derivative.
The Role of the Dienophile and Diene
-
Diene: The diene must be in the s-cis conformation to participate in the reaction. This conformation allows the terminal carbons of the diene to approach the dienophile simultaneously. If the diene is locked in the s-trans conformation, the reaction will not proceed readily.
-
Dienophile: The dienophile can be a variety of electron-deficient alkenes or alkynes. Electron-withdrawing groups (EWGs) on the dienophile increase the reaction rate by lowering the LUMO energy of the dienophile, making it a better acceptor of electrons from the diene's HOMO. Examples of EWGs include carbonyl groups, nitriles, and nitro groups.
Concerted Nature and Stereochemistry
The concerted nature of the Diels-Alder reaction is crucial for understanding the stereochemistry of the product. The stereochemistry of both the diene and dienophile is largely preserved in the product. This means that cis substituents on the dienophile remain cis in the product, and trans substituents remain trans. Similarly, the stereochemistry of the diene's substituents is also retained. This characteristic is extremely helpful in designing syntheses that need specific stereochemical outcomes.
Predicting the Regioselectivity of the Diels-Alder Reaction
Regioselectivity refers to the orientation of the substituents in the product. In unsymmetrical Diels-Alder reactions, there are two possible regioisomers. Predicting the major regioisomer is crucial for determining the correct product structure.
The "Ortho" and "Para" Relationship
The regioselectivity is often predicted using the following guidelines:
-
Electron-Rich Diene and Electron-Poor Dienophile: The major product is formed by placing the electron-donating group (EDG) on the diene ortho to the electron-withdrawing group (EWG) on the dienophile. This maximizes orbital overlap and lowers the energy of the transition state.
-
Electron-Poor Diene and Electron-Rich Dienophile: This scenario is less common but follows a similar principle: The EWG on the diene and EDG on the dienophile will preferentially orient ortho to each other.
Example: Consider the reaction between 1-methoxy-1,3-butadiene (electron-rich diene) and acrylonitrile (electron-poor dienophile). The major product will have the methoxy group ortho to the nitrile group.
Predicting the Stereoselectivity of the Diels-Alder Reaction
Stereoselectivity refers to the formation of one stereoisomer over another. Diels-Alder reactions often exhibit high stereoselectivity, particularly in the endo/exo selectivity.
Endo and Exo Selectivity
The endo product is formed when the substituents on the dienophile are oriented towards the newly formed bridgehead carbons, while the exo product has the substituents oriented away from the bridgehead carbons.
-
Endo Rule: The endo product is usually favored, although the energy difference between the endo and exo transition states can be small. This preference is explained by secondary orbital interactions (secondary orbital overlap) between the diene and the dienophile in the transition state leading to the endo product.
-
Steric Effects: In some cases, steric interactions can override the endo rule. If bulky substituents are present on either the diene or dienophile, the exo product may become favored.
Example: In the Diels-Alder reaction of cyclopentadiene with maleic anhydride, the endo product is significantly favored due to secondary orbital interactions.
Drawing the Correct Product: A Step-by-Step Approach
Let's illustrate how to systematically draw the correct product for a Diels-Alder reaction. Consider the reaction between 1,3-butadiene and maleic anhydride:
-
Identify the Diene and Dienophile: In this example, 1,3-butadiene is the diene, and maleic anhydride is the dienophile.
-
Determine the Orientation: The reaction is symmetrical, so there is no regioselectivity issue.
-
Consider Stereochemistry: Maleic anhydride has a cis configuration. This cis configuration will be preserved in the product.
-
Draw the Cyclic Structure: Form a six-membered ring by connecting the diene and dienophile. The stereochemistry should reflect the initial configuration of the reactants. Remember that the Diels-Alder reaction is a concerted [4+2] cycloaddition.
-
Check for Endo/Exo: In this symmetrical case, the endo product would mean having the anhydride carbonyl oxygens pointing towards the bridgehead carbons, while the exo product would have them pointing away. Because there is no significant steric hindrance, the endo product is expected.
Common Mistakes to Avoid
-
Ignoring Stereochemistry: Failing to account for the stereochemistry of the starting materials is a frequent mistake. Remember that the reaction is stereospecific.
-
Incorrect Regiochemistry: Misjudging the regiochemistry in unsymmetrical reactions is another common error. Apply the rules discussed earlier to determine the major regioisomer correctly.
-
Overlooking Endo/Exo Selectivity: Ignoring the endo/exo preference can lead to an incorrect product assignment.
-
Forgetting the s-cis Conformation: Remember that the diene must be in the s-cis conformation. If the diene is part of a larger ring structure, you need to check for the possibility of such conformations.
-
Incorrect Bond Formation: Ensure that you connect the atoms correctly during the formation of the six-membered ring. It should reflect the flow of electrons between the diene and the dienophile.
Advanced Diels-Alder Reactions
This basic understanding forms a foundation for more complex Diels-Alder reactions. Factors such as catalyst usage (Lewis acid catalysis can influence regio- and stereoselectivity dramatically) and inverse electron demand Diels-Alder reactions (where electron-rich dienophiles react with electron-poor dienes) add another layer of complexity, but the fundamental principles remain the same.
Conclusion: Mastering the Diels-Alder Reaction
Mastering the Diels-Alder reaction is a significant step in becoming proficient in organic chemistry. By understanding the mechanism, regioselectivity, stereoselectivity, and common pitfalls, you can accurately predict and draw the correct product for a wide range of Diels-Alder reactions. Remember to systematically analyze the reactants, apply the pertinent rules, and carefully draw the product's stereochemistry to successfully navigate this powerful reaction. Through consistent practice and a methodical approach, predicting the products of Diels-Alder reactions will become second nature.
Latest Posts
Latest Posts
-
A Nurse Is Preparing To Administer Esomeprazole 40 Mg
Mar 15, 2025
-
A Bank Reconciliation Should Be Prepared Periodically Because
Mar 15, 2025
-
What Are Functions Of Motor Movements In The Alimentary Canal
Mar 15, 2025
-
Who Must Inspect A Pfas And How Often
Mar 15, 2025
-
What If Rna Polymerase To Bind More Tightly Than Normal
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Draw The Correct Product For The Given Diels Alder Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
