Draw An Alkyl Halide That Would Undergo An Sn2 Reaction
Holbox
Mar 18, 2025 · 6 min read
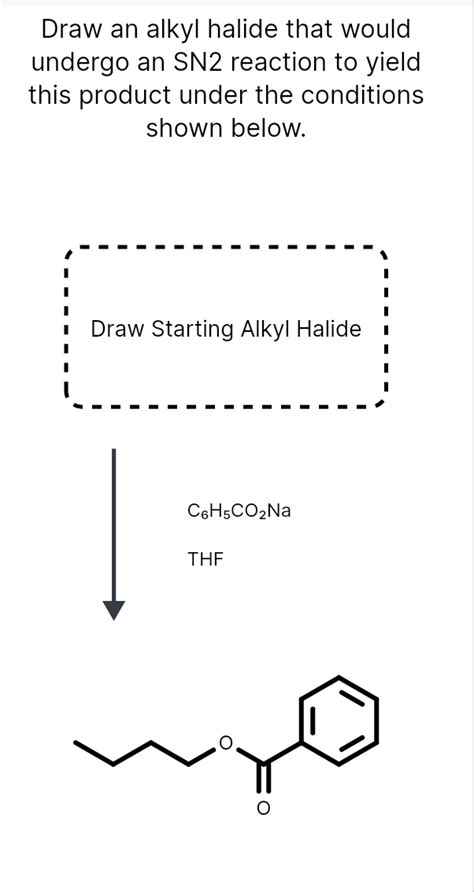
Table of Contents
Choosing the Right Alkyl Halide for an SN2 Reaction: A Deep Dive
The SN2 reaction, a cornerstone of organic chemistry, involves a nucleophilic attack on an alkyl halide, resulting in a substitution reaction with inversion of configuration. However, not all alkyl halides are created equal when it comes to their susceptibility to SN2 reactions. Steric hindrance, the solvent, and the nucleophile all play crucial roles in determining the success of the reaction. This article will delve deep into the factors influencing SN2 reactions and guide you in selecting the appropriate alkyl halide.
Understanding the SN2 Mechanism
Before diving into substrate selection, let's refresh our understanding of the SN2 mechanism. The reaction proceeds through a concerted mechanism, meaning the bond breaking and bond formation occur simultaneously. The nucleophile attacks the carbon atom bearing the halogen from the backside, leading to a transition state where the nucleophile and the leaving group are partially bonded to the carbon. This backside attack results in the inversion of configuration at the stereocenter, a hallmark of the SN2 reaction.
Key Factors Affecting SN2 Reactivity
Several factors influence the rate and feasibility of an SN2 reaction. These factors primarily revolve around the steric environment around the carbon atom bearing the halogen (the alpha carbon) and the nature of the nucleophile and solvent.
-
Steric Hindrance: This is arguably the most significant factor. Bulky substituents on the alpha carbon hinder the approach of the nucleophile, significantly slowing down or even preventing the SN2 reaction. The order of reactivity generally follows: methyl > primary > secondary >> tertiary. Tertiary alkyl halides essentially do not undergo SN2 reactions due to extreme steric hindrance.
-
Nature of the Leaving Group: A good leaving group is crucial. Leaving groups are typically weak bases that can stabilize the negative charge after they depart. Common good leaving groups include halides (I⁻ > Br⁻ > Cl⁻ > F⁻), tosylates (OTs), and mesylates (OMs). The better the leaving group, the faster the reaction.
-
Strength of the Nucleophile: A strong nucleophile is essential for a successful SN2 reaction. Strong nucleophiles are typically negatively charged or have lone pairs of electrons that are readily available for donation. The nucleophilicity is influenced by factors like charge, electronegativity, and steric hindrance.
-
Solvent Effects: The solvent plays a significant role. Polar aprotic solvents, such as DMF (dimethylformamide), DMSO (dimethyl sulfoxide), and acetone, are preferred for SN2 reactions. These solvents solvate the cation (e.g., Na⁺) more effectively than the nucleophile, leaving the nucleophile relatively "naked" and more reactive. Protic solvents, on the other hand, solvate both the cation and the nucleophile, reducing the nucleophile's reactivity and favoring SN1 reactions.
Choosing the Right Alkyl Halide: Examples and Explanations
Let's illustrate the principles discussed above with specific examples of alkyl halides and their suitability for SN2 reactions.
1. Methyl Halides (CH₃X):
These are the most reactive alkyl halides in SN2 reactions. The absence of any substituents on the alpha carbon allows for unimpeded nucleophilic attack.
- Example: Chloromethane (CH₃Cl) readily undergoes SN2 reactions with a wide range of nucleophiles.
2. Primary Alkyl Halides (RCH₂X):
Primary alkyl halides are also relatively reactive in SN2 reactions. They have only one substituent on the alpha carbon, leading to less steric hindrance compared to secondary or tertiary alkyl halides. However, the size of the R group can influence the reaction rate. Larger R groups lead to increased steric hindrance and slower reaction rates.
- Example: 1-bromobutane (CH₃CH₂CH₂CH₂Br) undergoes SN2 reactions efficiently. However, a primary alkyl halide with a bulky substituent like neopentyl bromide ((CH₃)₃CCH₂Br) would react much slower due to significant steric hindrance.
3. Secondary Alkyl Halides (R₂CHX):
Secondary alkyl halides are less reactive than primary alkyl halides in SN2 reactions due to increased steric hindrance. The presence of two substituents on the alpha carbon hinders the nucleophile's approach. While they can still undergo SN2 reactions under appropriate conditions (strong nucleophiles and polar aprotic solvents), the reaction rates are significantly slower compared to primary or methyl halides. Competition with SN1 reactions also becomes more significant.
- Example: 2-bromopropane (CH₃CHBrCH₃) can undergo SN2 reactions, but the rate will be considerably slower than that of 1-bromopropane.
4. Tertiary Alkyl Halides (R₃CX):
Tertiary alkyl halides are essentially unreactive in SN2 reactions. The three bulky substituents on the alpha carbon completely block the backside attack by the nucleophile. Instead, they predominantly undergo SN1 reactions.
- Example: 2-chloro-2-methylpropane ((CH₃)₃CCl) will not undergo SN2 reaction; SN1 is the favored pathway.
Illustrative Examples with Detailed Mechanisms
Let's illustrate the SN2 reaction with specific examples, detailing the mechanism and highlighting the crucial role of steric hindrance.
Example 1: Reaction of Chloromethane with Hydroxide Ion (Strong Nucleophile)
The reaction of chloromethane with hydroxide ion in a polar aprotic solvent like DMSO proceeds readily via SN2 mechanism.
CH₃Cl + OH⁻ -----> CH₃OH + Cl⁻
The hydroxide ion attacks the carbon atom from the backside, simultaneously breaking the C-Cl bond and forming the C-OH bond. The reaction proceeds with inversion of configuration (though chloromethane is achiral, the principle applies to chiral substrates).
Example 2: Reaction of 1-bromobutane with Sodium Iodide (Strong Nucleophile)
The reaction of 1-bromobutane with sodium iodide in acetone (polar aprotic solvent) is another classic example of an SN2 reaction.
CH₃CH₂CH₂CH₂Br + I⁻ -----> CH₃CH₂CH₂CH₂I + Br⁻
Iodide is a strong nucleophile, and the primary alkyl halide allows for relatively facile backside attack. The reaction proceeds smoothly with inversion of configuration if the substrate is chiral.
Example 3: A Less Favourable SN2 Reaction: 2-chloropentane with Methoxide
2-chloropentane, a secondary alkyl halide, reacts much slower with methoxide (CH₃O⁻) compared to the previous examples. The increased steric hindrance from the two alkyl groups on the alpha carbon slows the nucleophilic attack. While the reaction might still occur under vigorous conditions, it will compete with elimination reactions (E2) and likely proceed at a much slower rate.
CH₃CH₂CH₂CH(Cl)CH₃ + CH₃O⁻ -----> (Slow SN2 and competing E2) CH₃CH₂CH₂CH(OCH₃)CH₃ + HCl/CH₃CH₂CH=CHCH₃ + CH₃OH + Cl⁻
Conclusion: Strategic Substrate Selection for Successful SN2 Reactions
The success of an SN2 reaction hinges heavily on the careful selection of the alkyl halide. Prioritizing methyl and primary alkyl halides significantly increases the likelihood of a smooth and efficient reaction. While secondary alkyl halides can participate in SN2 reactions, their reactivity is considerably lower and often competes with elimination reactions. Tertiary alkyl halides are practically inert towards SN2 reactions due to overwhelming steric hindrance. By understanding these factors and selecting the appropriate alkyl halide, chemists can effectively manipulate the SN2 reaction to synthesize a wide array of valuable organic compounds. The choice of nucleophile and solvent are equally critical and must be considered in conjunction with the alkyl halide selection to optimize reaction conditions and yield. This detailed understanding ensures a more predictable and successful SN2 reaction outcome in the laboratory.
Latest Posts
Latest Posts
-
Use The Words To Fill In The Sentences Describing Anatomical Position
Mar 18, 2025
-
Because Of The Free Rider Problem
Mar 18, 2025
-
Within Mindbridge What Is A Control Point
Mar 18, 2025
-
The Percent Frequency Of A Class Is Computed By
Mar 18, 2025
-
Under What Circumstances Should A Companys Management Team
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Draw An Alkyl Halide That Would Undergo An Sn2 Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
