Density Of Water At 23 Degrees C
Holbox
Mar 16, 2025 · 5 min read
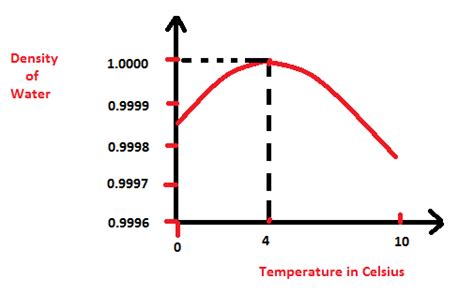
Table of Contents
Density of Water at 23 Degrees Celsius: A Comprehensive Guide
The density of water, a seemingly simple property, plays a crucial role in numerous scientific disciplines and everyday life. Understanding its behavior, particularly at specific temperatures like 23°C, is essential for accurate calculations and predictions in various fields, from oceanography and meteorology to chemistry and engineering. This comprehensive guide delves into the density of water at 23°C, exploring its value, influencing factors, applications, and implications.
Understanding Water Density
Density, denoted by the Greek letter ρ (rho), is defined as mass per unit volume. For water, this is typically expressed in kilograms per cubic meter (kg/m³) or grams per cubic centimeter (g/cm³). The density of water isn't constant; it's influenced by several factors, most notably temperature and pressure.
At standard atmospheric pressure (1 atm), water exhibits its maximum density at approximately 4°C (39.2°F), which is 999.97 kg/m³. As temperature increases or decreases from this point, the density decreases. This unusual behavior is due to the unique hydrogen bonding structure of water molecules.
Density of Water at 23°C: The Value
The precise density of water at 23°C and standard atmospheric pressure is approximately 997.7 kg/m³ or 0.9977 g/cm³. This value is slightly lower than the maximum density at 4°C due to the increased kinetic energy of water molecules at higher temperatures. The increased molecular motion leads to greater intermolecular spacing, resulting in a less dense structure.
While this value is widely accepted, minor variations can occur depending on the purity of the water sample and the precision of the measurement techniques used. Impurities, such as dissolved salts or gases, can slightly alter the density. Similarly, variations in atmospheric pressure can also cause subtle changes.
Factors Affecting Water Density at 23°C
Several factors contribute to the variation in water density, even at a seemingly fixed temperature like 23°C:
1. Temperature:
As discussed earlier, temperature is the most significant factor influencing water density. Even minor temperature fluctuations around 23°C will result in detectable changes in density. Accurate temperature control is therefore crucial for precise density measurements.
2. Pressure:
Pressure also affects water density, though to a lesser extent than temperature at typical environmental pressures. Increased pressure forces water molecules closer together, resulting in a slight increase in density. This effect is more pronounced at higher pressures.
3. Salinity:
The presence of dissolved salts in water, commonly expressed as salinity, significantly increases its density. Seawater, for example, is denser than freshwater due to the dissolved salts. The salinity of a water sample at 23°C directly influences its overall density.
4. Dissolved Gases:
Dissolved gases, such as oxygen and carbon dioxide, can also influence water density, albeit to a smaller degree than salinity. The concentration of dissolved gases varies depending on environmental factors like temperature and pressure.
5. Isotopic Composition:
Water molecules consist of hydrogen and oxygen atoms. However, variations in the isotopic composition of these atoms (e.g., the presence of deuterium instead of ordinary hydrogen) can subtly alter the overall density of the water. This effect is typically minor but can be relevant in precise scientific measurements.
Applications of Water Density at 23°C
The knowledge of water density at 23°C finds applications in numerous fields:
1. Oceanography:
Oceanographers utilize water density data to understand ocean currents, stratification, and mixing processes. Variations in density due to temperature and salinity drive ocean circulation patterns, influencing global climate and marine ecosystems. Accurate density measurements are essential for modeling ocean dynamics.
2. Meteorology:
In meteorology, water density is critical for understanding atmospheric processes, especially cloud formation and precipitation. The density of water vapor affects atmospheric stability and influences weather patterns.
3. Hydrology:
Hydrologists employ water density data to study the movement of water in rivers, lakes, and aquifers. Understanding water density helps in managing water resources and predicting water flow patterns.
4. Chemistry and Biochemistry:
In chemistry and biochemistry, water density is crucial for various laboratory procedures, including solution preparation, titration, and density measurements of other substances. Accurate density information is essential for precise experimental results.
5. Engineering:
Engineers use water density information in designing and operating various systems, including pipelines, cooling systems, and hydraulic equipment. Accurate density data is critical for ensuring the efficient and safe operation of these systems.
Measuring Water Density at 23°C
Several methods exist for measuring water density at 23°C, ranging from simple techniques to highly sophisticated instruments:
1. Hydrometer:
A hydrometer is a simple instrument used to measure the relative density of liquids. It floats in the liquid, and the depth to which it sinks indicates the liquid's density. While not highly precise, hydrometers are suitable for quick estimations.
2. Pycnometer:
A pycnometer is a precise glass instrument used for measuring liquid density. It consists of a small bottle with a tightly fitting stopper, which includes a capillary tube to allow for precise volume control. The mass of a known volume of water is measured to determine its density. Pycnometers provide highly accurate density measurements.
3. Digital Density Meter:
Digital density meters utilize advanced techniques, such as oscillating U-tube technology, to provide highly accurate and rapid density measurements. These instruments offer high precision and automated data recording.
Conclusion
The density of water at 23°C, while seemingly a specific value, holds significant importance across a vast range of scientific disciplines and practical applications. Understanding its value and the factors that influence it is essential for accurate calculations, predictions, and informed decision-making in numerous fields. From oceanographic models to chemical experiments and engineering designs, precise knowledge of water density at this temperature remains a cornerstone of various crucial applications. The methods for measuring water density, from simple hydrometers to advanced digital instruments, ensure accurate data acquisition for diverse needs. Further research into the intricacies of water density continues to refine our understanding of this fundamental property and its implications for the world around us. Continued exploration into the nuances of water density at various temperatures and pressures will undoubtedly yield further insights and advancements in diverse fields. Accurate determination and understanding of this critical property remain crucial for progress across scientific disciplines and engineering applications.
Latest Posts
Latest Posts
-
A Process Cost Accounting System Is Most Appropriate When
Mar 17, 2025
-
An Example Of A Breach Of Ephi Is
Mar 17, 2025
-
What Is Involved In Safety Monitoring
Mar 17, 2025
-
Which Of The Following Best Describes The Term Cleavage
Mar 17, 2025
-
When Prioritizing Six Sigma Projects Within An Organization
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Density Of Water At 23 Degrees C . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
