Consider The Reaction Of 2-methyl-1 3-cyclohexadiene With Hcl
Holbox
Mar 17, 2025 · 6 min read
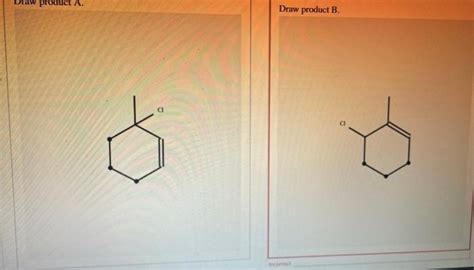
Table of Contents
Considering the Reaction of 2-Methyl-1,3-Cyclohexadiene with HCl: A Deep Dive into Electrophilic Addition
The reaction between 2-methyl-1,3-cyclohexadiene and HCl offers a fascinating case study in electrophilic addition reactions, showcasing the interplay of regioselectivity, carbocation stability, and potential rearrangements. This article will delve into the intricacies of this reaction, exploring the mechanistic pathways, predicting the major and minor products, and discussing the factors influencing product distribution. We'll also consider the implications of this reaction within a broader context of organic chemistry principles.
Understanding the Reactants
Before diving into the reaction mechanism, let's briefly examine the properties of the reactants:
2-Methyl-1,3-Cyclohexadiene
This molecule is a conjugated diene, meaning it possesses two double bonds separated by a single bond. This conjugation significantly impacts its reactivity. The delocalized π electrons make the diene more susceptible to electrophilic attack compared to isolated alkenes. The methyl group at the 2-position introduces steric hindrance and influences the regioselectivity of the addition.
Hydrogen Chloride (HCl)
HCl acts as a strong electrophile in this reaction, providing a proton (H+) that initiates the electrophilic addition. The chloride ion (Cl-) acts as a nucleophile in the subsequent step. The strength of HCl as an electrophile ensures a relatively fast reaction rate.
The Reaction Mechanism: A Step-by-Step Approach
The reaction proceeds via a two-step mechanism, characteristic of electrophilic additions to conjugated dienes:
Step 1: Electrophilic Attack and Carbocation Formation
The reaction begins with the electrophilic attack of the proton (H+) from HCl on one of the double bonds in 2-methyl-1,3-cyclohexadiene. There are two possible sites for the initial protonation: at carbon 1 or carbon 3. Protonation at carbon 1 leads to the formation of a resonance-stabilized allylic carbocation. Protonation at carbon 3 also forms a resonance-stabilized allylic carbocation, but the structure differs.
Note: The stability of the resulting carbocation is the key determinant of the regioselectivity. Allylic carbocations, those where the positive charge is adjacent to a double bond, are significantly more stable than typical secondary or primary carbocations due to resonance stabilization.
The resonance structures for the carbocation formed by protonation at carbon 1 are:
(Image: Show resonance structures of allylic carbocation formed from protonation at C1. Clearly label carbons and charges.)
Similarly, the resonance structures for the carbocation formed from protonation at carbon 3 are:
(Image: Show resonance structures of allylic carbocation formed from protonation at C3. Clearly label carbons and charges.)
Analyzing these resonance structures, we can see that both carbocations are stabilized through resonance. However, subtle differences in substitution influence their relative stability. The carbocation resulting from protonation at C1 is slightly more stable due to the inductive effect of the methyl group.
Step 2: Nucleophilic Attack and Product Formation
In the second step, the chloride ion (Cl-) acts as a nucleophile, attacking the positively charged carbon of the allylic carbocation. Since the carbocation is resonance-stabilized, the nucleophile can attack at either of the positive charge locations. This leads to the formation of two different products:
(Image: Show the two possible products resulting from nucleophilic attack at different positions of the allylic carbocation. Clearly label each product and indicate their major/minor status based on the stability of the preceding carbocations.)
The major product will be derived from the more stable carbocation (from the initial protonation at C1). This product will have the chlorine atom attached to the carbon bearing the methyl group. The minor product will arise from the less stable carbocation (from the initial protonation at C3).
Factors Influencing Product Distribution
Several factors contribute to the observed ratio of major and minor products:
- Carbocation Stability: As discussed above, the stability of the intermediate carbocations is paramount. The more stable carbocation leads to a higher proportion of the corresponding product.
- Steric Effects: The methyl group introduces steric hindrance, making the attack on the more substituted carbon slightly less favorable. However, this effect is often overshadowed by the effect of carbocation stability.
- Kinetic vs. Thermodynamic Control: The reaction conditions (temperature, solvent) can influence whether kinetic or thermodynamic control dominates. At lower temperatures, the kinetic product (formed faster) may be favored. At higher temperatures, the thermodynamic product (more stable) might predominate.
Predicting the Products: A Deeper Look at Regioselectivity and Stereochemistry
The reaction exhibits both regioselectivity and, to a lesser extent, stereoselectivity.
Regioselectivity
The major product will be the 1-chloro-2-methyl-3-cyclohexene, resulting from the attack of Cl- on the more stable carbocation. The minor product will be 3-chloro-2-methyl-3-cyclohexene. The difference in product yields reflects the difference in stability of the respective carbocations.
Stereochemistry
The addition of HCl to the double bond can lead to both cis and trans isomers, but the degree of stereoselectivity is typically low. The reaction is not highly stereospecific because the carbocation intermediate allows for rotation around the C-C bond before nucleophilic attack.
1,2 vs. 1,4 Addition
In the reaction of HCl with a conjugated diene, both 1,2- and 1,4-addition products can be formed. 1,2-addition refers to the addition of H and Cl across adjacent carbons, whereas 1,4-addition involves the addition across carbons separated by one carbon atom. In this reaction with 2-methyl-1,3-cyclohexadiene, the 1,2-addition products are more favoured due to their kinetic stability.
Experimental Considerations and Applications
The reaction between 2-methyl-1,3-cyclohexadiene and HCl can be performed under relatively mild conditions. Typically, the reaction is carried out in a non-polar solvent to prevent the formation of unwanted side products. The products can be analyzed using techniques like Gas Chromatography-Mass Spectrometry (GC-MS) or Nuclear Magnetic Resonance (NMR) spectroscopy.
Broader Context and Related Reactions
The reaction of 2-methyl-1,3-cyclohexadiene with HCl provides a valuable example of electrophilic addition to conjugated dienes. Understanding this reaction is crucial for grasping concepts such as:
- Electrophilic Aromatic Substitution: While this reaction is electrophilic addition, the underlying principles of electrophilic attack and carbocation stability are relevant to electrophilic aromatic substitution.
- Diels-Alder Reaction: Conjugated dienes like 2-methyl-1,3-cyclohexadiene are key participants in the Diels-Alder reaction, a cycloaddition reaction of significant synthetic utility.
- Allylic Rearrangements: The formation of resonance-stabilized allylic carbocations opens the door to understanding allylic rearrangements, where the double bond shifts position during a reaction.
This reaction serves as a foundational concept in organic chemistry, reinforcing the importance of understanding reaction mechanisms, predicting product formation, and considering the various factors influencing reaction outcomes. Mastering this reaction solidifies a strong understanding of fundamental organic chemistry principles and prepares the student to tackle more complex reaction systems.
Conclusion
The reaction of 2-methyl-1,3-cyclohexadiene with HCl is a rich example of electrophilic addition to a conjugated diene. The mechanistic pathways, product distribution, and the factors influencing regioselectivity provide valuable insights into the behavior of conjugated systems. This detailed exploration highlights the importance of understanding carbocation stability, resonance structures, and the impact of both steric and electronic effects in organic chemistry reactions. This knowledge lays a solid foundation for further exploration into more complex organic reactions and synthetic pathways.
Latest Posts
Latest Posts
-
Productive Efficiency Occurs At The Point Where
Mar 17, 2025
-
A Multiple Step Income Statement Reports Multiple Levels Of
Mar 17, 2025
-
Where Is The Tissue Pictured Found
Mar 17, 2025
-
Per Navsup P 805 What Does This Indicator Show
Mar 17, 2025
-
Which Of The Following Can Be Translated Into Protein
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Consider The Reaction Of 2-methyl-1 3-cyclohexadiene With Hcl . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
